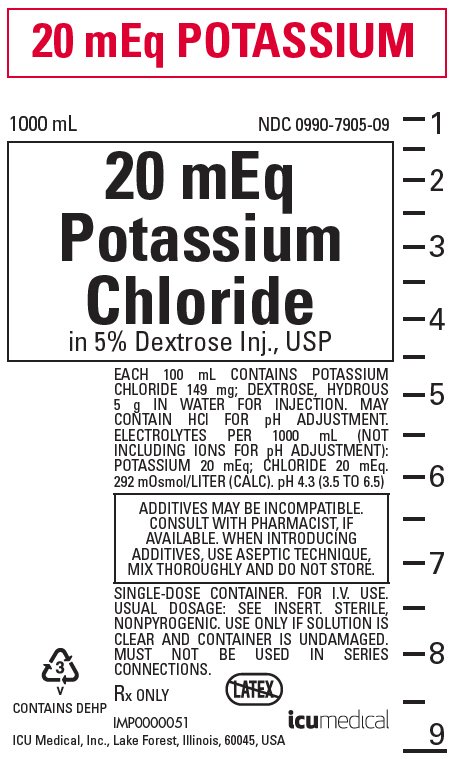
Na2o is what kind of chemical reaction
Na2o Is What Kind Of Chemical Reaction. Ch3o formal charge. In contrast the class F fly ash non-cement mortars exhibited better efficacy than class C when cured at high. This observation is reflected in Daltons postulate that atoms cannot be _____ or destroyed or changed into other types of atoms. That is it always remains constant.
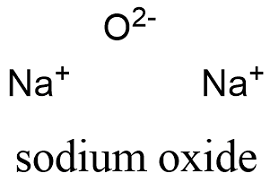 Sodium Oxide Formula From softschools.com
Sodium Oxide Formula From softschools.com
According to this law matter can not be created nor destroyed in a chemical reaction. Like in all chemical reactions the total mass of products is equal to the total mass of reactants. This observation is reflected in Daltons postulate that atoms cannot be _____ or destroyed or changed into other types of atoms. A solution containing 253 mL of 01065 N HCl is added to one containing 922 mL of 02715 M H 2SO 4 and 50 mL of 100 N KOH are added. If the substance dissolves give the type of dissolving process involved. Answer 1 of 6.
Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
Answer 1 of 6. According to this law matter can not be created nor destroyed in a chemical reaction. Table 1 shows the chemical composition of the class F fly ash used in current study as an aluminosilicate precursor tested according to ASTM-C618. A formula like CuCl25H2O takes less than a second on a TI-84 Plus and even accepts hydrates and nested parentheses. C7H16 11O2 7CO2. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant.
 Source: coolgyan.org
Source: coolgyan.org
If the substance dissolves give the type of dissolving process involved. C7H16 11O2 7CO2. A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. Its percent composition molar mass solubility and if its a hydrocarbon its complete balanced combustion reaction equation. Predict the reaction products when potassium hydroxide is added to water by writing a balanced chemical equation.
 Source: youtube.com
Source: youtube.com
Answer 1 of 6. A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. Table 1 shows the chemical composition of the class F fly ash used in current study as an aluminosilicate precursor tested according to ASTM-C618. FDE 201-LECTURE NOTES-66 In general we can write a valid stoichiometric chemical reaction in a general form as follows Normally since the reactants are consumed while the products are generated as the reaction proceeds the coefficients of reactants are of negative sign products are of positive sign For instance for the valid chemical reaction. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction.
 Source: slideplayer.com
Source: slideplayer.com
It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. CuNO32 aq 2NaOH aq CuOH2 s 2NaNO3 aq aq means that it is in solution aquesous s - means that it is a solid. C7H16 11O2 7CO2. If the substance dissolves give the type of dissolving process involved. Mass is conserved in a chemical reaction.
 Source: youtube.com
Source: youtube.com
According to this law matter can not be created nor destroyed in a chemical reaction. A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. The reaction that occurs is shown below. Table 1 shows the chemical composition of the class F fly ash used in current study as an aluminosilicate precursor tested according to ASTM-C618. That is it always remains constant.
 Source: youtube.com
Source: youtube.com
A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. Like in all chemical reactions the total mass of products is equal to the total mass of reactants. Since each type of atom has its own _____ atomic mass the total mass cannot change in a chemical reaction. Answer 1 of 6. In a chemical equation the number of molecules or formula units of a particular substance that are participating in the chemical reaction is indicated by a.
 Source: youtube.com
Source: youtube.com
Substrate CHEMICAL ENGINEERING REVIEW ANALYTICAL CHEMISTRY 2. The equation given below describes the accurate gas-phase PA and intrinsic GB of a base B here defined as B XM n1 OH and. It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. For example-2H2 O2 2 H2O Reactants Product 4 2 x 1632 2 x 18 36 g.
 Source: youtube.com
Source: youtube.com
If the substance dissolves give the type of dissolving process involved. A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. A small and very fast program that prompts you for any chemical formula and outputs everything you ever wanted to know about it. Answer 1 of 6. The reaction that occurs is shown below.
 Source: youtube.com
Source: youtube.com
A formula like CuCl25H2O takes less than a second on a TI-84 Plus and even accepts hydrates and nested parentheses. Mass is conserved in a chemical reaction. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. This observation is reflected in Daltons postulate that atoms cannot be _____ or destroyed or changed into other types of atoms.
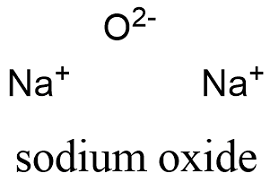 Source: softschools.com
Source: softschools.com
Since each type of atom has its own _____ atomic mass the total mass cannot change in a chemical reaction. In a chemical equation the number of molecules or formula units of a particular substance that are participating in the chemical reaction is indicated by a. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. If the substance dissolves give the type of dissolving process involved.
 Source: youtube.com
Source: youtube.com
For example-2H2 O2 2 H2O Reactants Product 4 2 x 1632 2 x 18 36 g. Ch3o formal charge. A hypothetical gas-phase protonation reaction has been expressed in eq 1 for evaluating the basicity of diverse XM n1 OH hydroxides of K- Na- and Li-related species. First please note that this reaction has very little practical valueI will get back to this. This observation is reflected in Daltons postulate that atoms cannot be _____ or destroyed or changed into other types of atoms.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title na2o is what kind of chemical reaction by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.