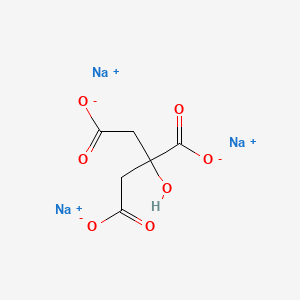
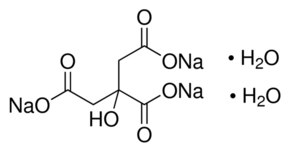
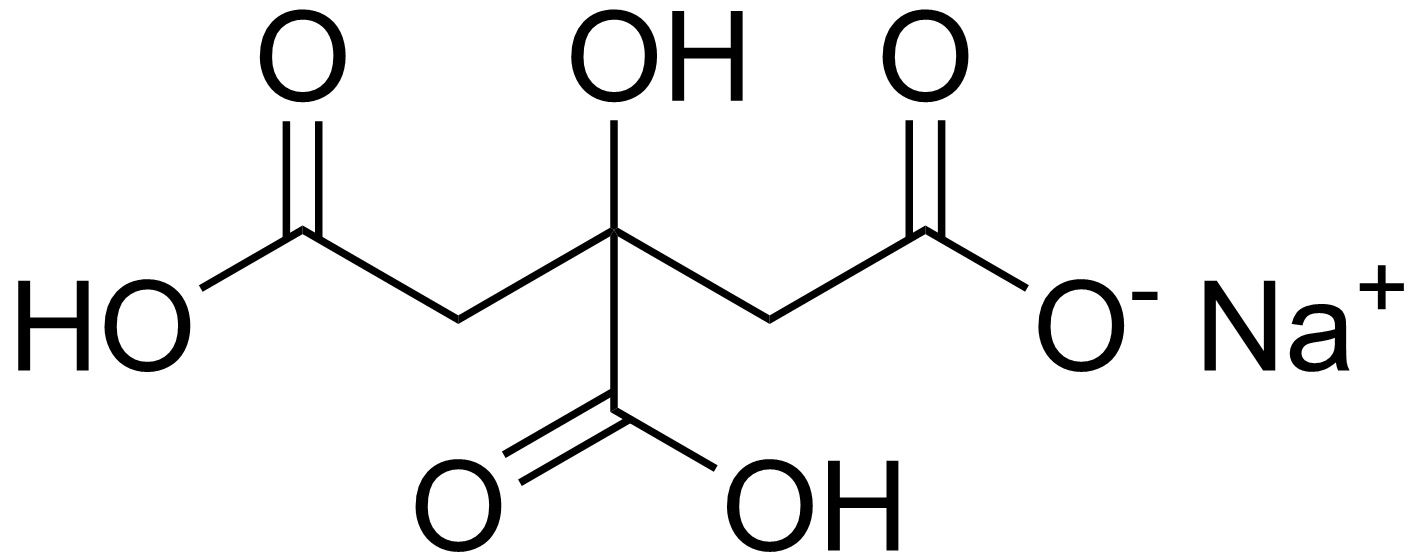
Na3c6h5o7 2h2o name
Na3c6h5o7 2h2o Name. A repeat comparison. 44 g of O2 a determine the limiting reagent b determine the number of Limiting Reactant Date _____ 1. The name or initials of the pharmacist who filled the order. 0 g of zinc and 30.
 Sodium Citrate Dihydrate Food Grade Manufacturer Supplier Exporter From annexechem.net
Sodium Citrate Dihydrate Food Grade Manufacturer Supplier Exporter From annexechem.net
The label has the name of the correct patient and physician. Chemistry archive june 19 2012 chegg limiting reactant from Limiting Reactant And Percent Yield Worksheet. Limiting reactant and percent yield practice worksheet answers. How many grams of NO are formed. And the number of refills remaining. A repeat comparison.
44 g of O2 a determine the limiting reagent b determine the number of Limiting Reactant Date _____ 1.
A repeat comparison. Calculate the percentage yield. 44 g of O2 a determine the limiting reagent b determine the number of Limiting Reactant Date _____ 1. Academiaedu is a platform for academics to share research papers. The label has the name of the correct patient and physician. The name or initials of the pharmacist who filled the order.
 Source: annexechem.net
Source: annexechem.net
And the number of refills remaining. The label has the name of the correct patient and physician. Step 2 determine the moles of co. Academiaedu is a platform for academics to share research papers. Ca oh 2 2hcl cacl2 2h2o a if you have spilled 6 3 mol of hcl and put 2 8 mol of ca oh 2 on it which substance is the limiting reactant.
 Source: alibaba.com
Source: alibaba.com
Additional label information andor auxiliary labels may be required according to good pharmacy practice and by federal and state law depending on the drug dispensed. The name or initials of the pharmacist who filled the order. Additional label information andor auxiliary labels may be required according to good pharmacy practice and by federal and state law depending on the drug dispensed. Ca oh 2 2hcl cacl2 2h2o a if you have spilled 6 3 mol of hcl and put 2 8 mol of ca oh 2 on it which substance is the limiting reactant. Calculate the percentage yield.

0 g Solution steps Step 1 Determine the moles of I 2 O 5 Step 2 Determine the moles of CO Step 3 Do a. How many grams of NO are formed. Limiting reactant and percent yield practice worksheet answers. The label has the name of the correct patient and physician. Step 2 determine the moles of co.
 Source: alibaba.com
Source: alibaba.com
How many grams of NO are formed. Ca oh 2 2hcl cacl2 2h2o a if you have spilled 6 3 mol of hcl and put 2 8 mol of ca oh 2 on it which substance is the limiting reactant. A repeat comparison. The correct drug name quantity and strength. 6 grams of CO produces 36.
Step 2 determine the moles of co. A repeat comparison. Additional label information andor auxiliary labels may be required according to good pharmacy practice and by federal and state law depending on the drug dispensed. The correct drug name quantity and strength. 6 grams of CO produces 36.
 Source: twellsansino.en.made-in-china.com
Source: twellsansino.en.made-in-china.com
6 grams of CO produces 36. And the number of refills remaining. 44 g of O2 a determine the limiting reagent b determine the number of Limiting Reactant Date _____ 1. Limiting reactant and percent yield practice worksheet answers. The correct drug name quantity and strength.
 Source: 21food.com
Source: 21food.com
The correct drug name quantity and strength. Calculate the percentage yield. Mole Mass and Particle Conversions 3. Step 2 determine the moles of co. Academiaedu is a platform for academics to share research papers.
 Source: njjyxw.en.made-in-china.com
Source: njjyxw.en.made-in-china.com
The correct drug name quantity and strength. Chemistry archive june 19 2012 chegg limiting reactant from Limiting Reactant And Percent Yield Worksheet. 6 grams of CO produces 36. How many grams of NO are formed. Mole Mass and Particle Conversions 3.
 Source: ereztech.com
Source: ereztech.com
0 g Solution steps Step 1 Determine the moles of I 2 O 5 Step 2 Determine the moles of CO Step 3 Do a. Mole Mass and Particle Conversions 3. Step 2 determine the moles of co. Calculate the percentage yield. The name or initials of the pharmacist who filled the order.
 Source: en-academic.com
Source: en-academic.com
Academiaedu is a platform for academics to share research papers. Ca oh 2 2hcl cacl2 2h2o a if you have spilled 6 3 mol of hcl and put 2 8 mol of ca oh 2 on it which substance is the limiting reactant. The correct drug name quantity and strength. A repeat comparison. Calculate the percentage yield.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title na3c6h5o7 2h2o name by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.