Naclo4 molar mass
Naclo4 Molar Mass. 3062 gmol 2780 gmol 3422 gmol 3152 gmol 1230 gmol. The combustion of 0136 kg of methane CH4 in the presence of excess oxygen produces 353 g of carbon dioxide. Academiaedu is a platform for academics to share research papers. Converting amu to grams.

Thus the R will be CH3 mol. Academiaedu is a platform for academics to share research papers. What is the molar mass of aluminum sulfate. What is the molar mass of laughing gas N2O. What is the percent yield. How many mole ratios can be correctly obtained from the balanced chemical equation 2Al2O3l 4Als 3O2g.
Academiaedu is a platform for academics to share research papers.
The empirical formula mass 911 gmol b 549 mL 00549 L mol NaOH 00549 L100 molL 00549 mol NaOH Because X has two acidic hydrogens two mol of NaOH are required to titrate 1 mol of X. How many molecules of sulfur trioxide are in 780 grams. Academiaedu is a platform for academics to share research papers. The combustion of 0136 kg of methane CH4 in the presence of excess oxygen produces 353 g of carbon dioxide. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N. The The pH of a solution ranges from 1-14 1-6 are acidic 7 is neutral and 8-14 are basic.
 Source: researchgate.net
Source: researchgate.net
A common mistake is to forget the subscript 2 outside the parentheses in NH42CO3 which could give a much lower molar mass. The combustion of 0136 kg of methane CH4 in the presence of excess oxygen produces 353 g of carbon dioxide. What is the molar mass of aluminum sulfate. Molar Mass of Frequently Calculated Chemicals. What is the mass of ammonium chloride is 5000 mL of a 0198 M solution.
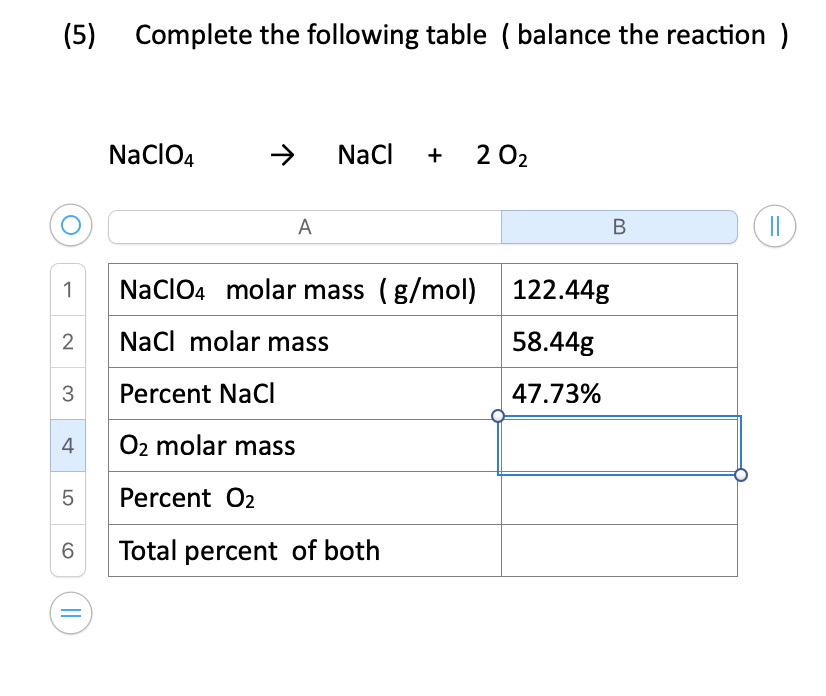 Source: bartleby.com
Source: bartleby.com
What is the percent yield. Molar mass of KNO3 101. The The pH of a solution ranges from 1-14 1-6 are acidic 7 is neutral and 8-14 are basic. Molar Mass of Frequently Calculated Chemicals. 1 mol X mol X 00549 mol NaOH x 00274 mol X 2 mol NaOH 500 g X molar mass 182 gmol 00274 mol Because the molar mass is twice the empirical formula mass the molecular formula is.
 Source: en.wikipedia.org
Source: en.wikipedia.org
A common mistake is to forget the subscript 2 outside the parentheses in NH42CO3 which could give a much lower molar mass. What is the percent yield. What is the molar mass of aluminum sulfate. Thus the R will be CH3 mol. Can you explain this answer.

Molar mass of KNO3 101. A common mistake is to forget the subscript 2 outside the parentheses in NH42CO3 which could give a much lower molar mass. Molar Mass of Frequently Calculated Chemicals. How many molecules of sulfur trioxide are in 780 grams. 2 with Azide was tested on trypticase soy agar for 24 hours 48 hours and 72 hours and was found to be negative for bacteria.
 Source: youtube.com
Source: youtube.com
Molar Mass of Frequently Calculated Chemicals. The combustion of 0136 kg of methane CH4 in the presence of excess oxygen produces 353 g of carbon dioxide. For C17H25N the molar mass 17C 25H 1N equals 24343 gmole and the three theoretical values for by weight are calculated as follows. How many molecules of sulfur trioxide are in 780 grams. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N.
 Source: en.wikipedia.org
Source: en.wikipedia.org
How many mole ratios can be correctly obtained from the balanced chemical equation 2Al2O3l 4Als 3O2g. 150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C. Now the molecular mass of COOC2 H5 is 72 thus the molecular mass of R will be 15 ie 88 72 16. If 201 1023 atoms of an element from Group IA of the periodic table has a mass of 7675 grams this element is most likely. Y on treatment with ethanol in presence of H2SO4 gives a pleasant smelling compound Z which should be an ester RCOOC2 H5 of molecular mass 88.
 Source: lab.honeywell.com
Source: lab.honeywell.com
What is the mass of ammonium chloride is 5000 mL of a 0198 M solution. 150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C. Converting amu to grams. Now the molecular mass of COOC2 H5 is 72 thus the molecular mass of R will be 15 ie 88 72 16. For C17H25N the molar mass 17C 25H 1N equals 24343 gmole and the three theoretical values for by weight are calculated as follows.
Acidic - Introduction to pH - the acidic and basic alkaline definition. What is the molar mass of laughing gas N2O. The The pH of a solution ranges from 1-14 1-6 are acidic 7 is neutral and 8-14 are basic. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. 3062 gmol 2780 gmol 3422 gmol 3152 gmol 1230 gmol.
 Source: researchgate.net
Source: researchgate.net
Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. The combustion of 0136 kg of methane CH4 in the presence of excess oxygen produces 353 g of carbon dioxide. Nh4ch3coo acid or base email protected. What is the percent yield.
 Source: chegg.com
Source: chegg.com
Since the mass is less than half the molar mass 4296 05 the number of formula units should be less than half Avogadros number 26 x I02360 x 1023 05. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. 2 with Azide was tested on trypticase soy agar for 24 hours 48 hours and 72 hours and was found to be negative for bacteria. If 201 1023 atoms of an element from Group IA of the periodic table has a mass of 7675 grams this element is most likely. 150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title naclo4 molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.