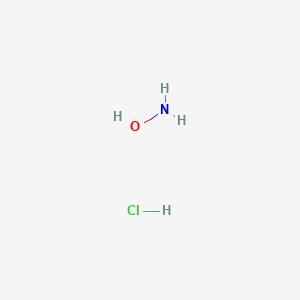
Nh2oh hcl molar mass
Nh2oh Hcl Molar Mass. 1 a 0b 1 c 0d H. Label Each Compound With a Variable a Fe b HCl c FeCl 2 d H 2 2. 0a 1 b 2 c 0d 3. There are two common techniques for balancing redox equations.
 Hydroxylamine Hydrochloride 5470 11 1 Tci America From tcichemicals.com
Hydroxylamine Hydrochloride 5470 11 1 Tci America From tcichemicals.com
1L 0100 mol NaOH 1 mol SA mol SA in 100 g SA 724 mL x x x 1000 mL 1L 1 mol NaOH 000724 mol SA 100 g 138 gmol SA molar mass 000724 mol Because the empirical formula mass and the molar mass. Thus the moles of morphine at the equivalence point was equal to the moles of HCl 00100 M000284 L 284105 mol. 0a 1 b 2 c 0d 3. Its molar mass is 102 gmol. The amount of arsenic pentasulphide that can be. The mass of AgCl in g obtained will be.
The amount of arsenic pentasulphide that can be.
Determine the molecular formula of cadaverine. Molar mass of AgCl 1435 g mol1 Main Online April 15 2018 I a 035 b 054 c 041 d 048 5. Fe HCl FeCl2 H2 1. 1 gram of a carbonate M2CO3 on treatment with excess HCl produces 001186 mole of CO2. Oxidation number change method ion-electron method also called the half-reaction method. Please use this book to increase your knowledge for the laboratory pratictioner.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Fe HCl FeCl2 H2 1. Fe HCl FeCl2 H2 1. The mass of morphine reacted was found by multiplying the moles reacted by the molar mass of morphine 0008094. Feb 10 2021 a the percent composition of hydrazoic acid HN₃ The mass of the atoms are as follows - mass of H 1 gmol mass of N 14 gmol The percentage of H mass of hydrogen mass of total compound 100 The percentage of H 1 1 3 14 100 2. 1 a 0b 1 c 0d H.
 Source: tcichemicals.com
Source: tcichemicals.com
Molar mass of AgCl 1435 g mol1 Main Online April 15 2018 I a 035 b 054 c 041 d 048 5. Oxidation number change method ion-electron method also called the half-reaction method. Enter the email address you signed up with and well email you a reset link. Eu não sei de nada. 0a 1 b 2 c 0d 3.
 Source: thomassci.com
Source: thomassci.com
The amount of arsenic pentasulphide that can be. Oxidation number change method ion-electron method also called the half-reaction method. The mass of AgCl in g obtained will be. B Unlike enthalpies of formation standard molar entropies of elements at the reference temperature of 298 K are not zero. 1 gram of a carbonate M2CO3 on treatment with excess HCl produces 001186 mole of CO2.
Oxidation number change method ion-electron method also called the half-reaction method. 1L 0100 mol NaOH 1 mol SA mol SA in 100 g SA 724 mL x x x 1000 mL 1L 1 mol NaOH 000724 mol SA 100 g 138 gmol SA molar mass 000724 mol Because the empirical formula mass and the molar mass. Create a System of Equations One Per Element Fe. 0a 1 b 0c 2 d Cl. The molar mass of M2CO3 in g mol1 is.
 Source: researchgate.net
Source: researchgate.net
0a 1 b 0c 2 d Cl. C Standard molar entropies generally increase with decreasing molar mass. Eu não sei de nada. 0a 1 b 0c 2 d Cl. Feb 10 2021 a the percent composition of hydrazoic acid HN₃ The mass of the atoms are as follows - mass of H 1 gmol mass of N 14 gmol The percentage of H mass of hydrogen mass of total compound 100 The percentage of H 1 1 3 14 100 2.

Determine the molecular formula of cadaverine. Oxidation number change method ion-electron method also called the half-reaction method. A The standard molar entropies of gases are greater than those of liquids and solids consistent with our interpretation of experimental observations. Feb 10 2021 a the percent composition of hydrazoic acid HN₃ The mass of the atoms are as follows - mass of H 1 gmol mass of N 14 gmol The percentage of H mass of hydrogen mass of total compound 100 The percentage of H 1 1 3 14 100 2. The molar mass of M2CO3 in g mol1 is.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Definition of hydrazoic acid in the Definitions. Dec 16 2009 Hydrazoic acid has been shown to be very reactive under these. Eu não sei de nada. The molar mass of M2CO3 in g mol1 is. 0a 1 b 2 c 0d 3.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Molar mass of AgCl 1435 g mol1 Main Online April 15 2018 I a 035 b 054 c 041 d 048 5. The amount of arsenic pentasulphide that can be. Fe HCl FeCl2 H2 1. 1 gram of a carbonate M2CO3 on treatment with excess HCl produces 001186 mole of CO2. Please use this book to increase your knowledge for the laboratory pratictioner.
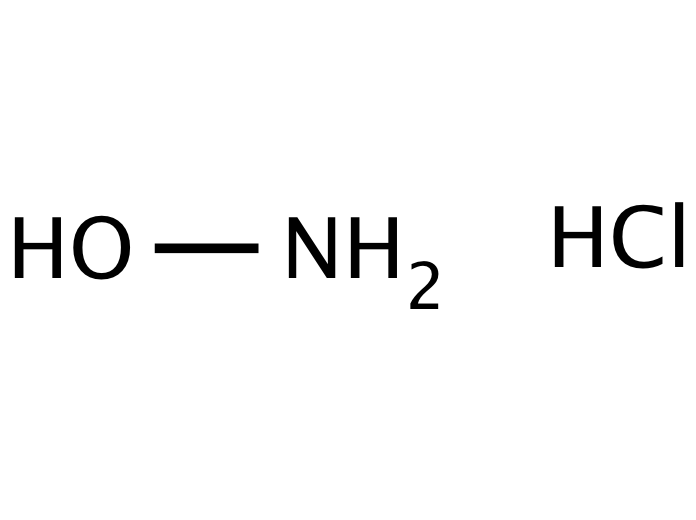 Source: discofinechem.com
Source: discofinechem.com
1L 0100 mol NaOH 1 mol SA mol SA in 100 g SA 724 mL x x x 1000 mL 1L 1 mol NaOH 000724 mol SA 100 g 138 gmol SA molar mass 000724 mol Because the empirical formula mass and the molar mass. Main 2017 a 1186 b 843 c 1186 d 1186 6. The molar mass of M2CO3 in g mol1 is. Thus the moles of morphine at the equivalence point was equal to the moles of HCl 00100 M000284 L 284105 mol. 0a 1 b 2 c 0d 3.
 Source: gbiosciences.com
Source: gbiosciences.com
Main 2017 a 1186 b 843 c 1186 d 1186 6. There are two common techniques for balancing redox equations. C Standard molar entropies generally increase with decreasing molar mass. 1 a 0b 1 c 0d H. The number of moles of morphine that reacted with HCl was calculated by assuming a 11 stoichiometric ratio.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title nh2oh hcl molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.