Nh4cl in water
Nh4cl In Water. First 5947 mg 3 mmol of 110-phenanthroline C 12 H 8 N 2 H 2 O was dissolved in 200 mL of hot. Hydrohen atoms of ammonium ion have a small positive charge. Aqueous ammonium chloride has weak acidic charactristics. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry.
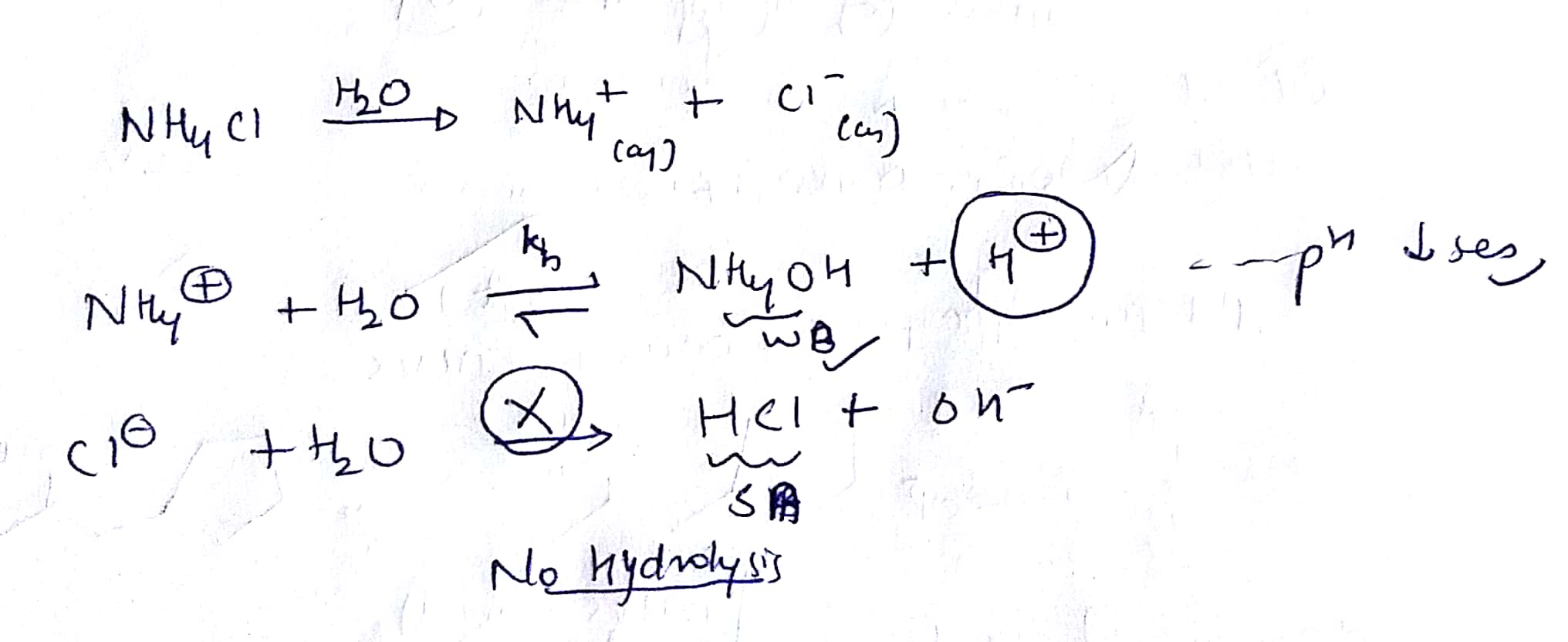 On Dissolution Of Nh4cl In Water Ph Of Water Decreases It Is Due To Hydrolysis Of Option 1 Nh4 Ion Option 2 Chloride Ion Option 3 Both Ammonium And Chloride Ion From embibe.com
On Dissolution Of Nh4cl In Water Ph Of Water Decreases It Is Due To Hydrolysis Of Option 1 Nh4 Ion Option 2 Chloride Ion Option 3 Both Ammonium And Chloride Ion From embibe.com
Identify X and Y giving the chemical equation of the reactions involved. Include the correct sign for the heat change. Aqueous ammonium chloride has weak acidic charactristics. First 5947 mg 3 mmol of 110-phenanthroline C 12 H 8 N 2 H 2 O was dissolved in 200 mL of hot. Calculate the heat absorbed by the water or the solution in J and convert it to kJ. Hydrohen atoms of ammonium ion have a small positive charge.
Ammonia sodium chloride NaCl and water are given as products when ammonium chloride is heated with aqueous sodium hydroxide NaOH aq.
Aqueous ammonium chloride has weak acidic charactristics. Include the correct sign for the heat change. X Chlorine Cl 2 Y Bleaching powder CaOCl2 CaOH2 s Cl2 g CaOCl2 s H2O Calcium oxychloride bleaching powder 40. Calculate the heat released by the reaction in terms of kJmol NH4Cl. Aqueous ammonium chloride has weak acidic charactristics. Ammonium salt and alkali reaction mechanism.
 Source: youtube.com
Source: youtube.com
In Part A the moles of NH4Cl are calculated from the mass of solid NH4Cl. Fill in the missing data in the following table. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. Improvements based on the literature were as follows. Ammonium salt and alkali reaction mechanism.
 Source: youtube.com
Source: youtube.com
Include the correct sign for the heat change. First 5947 mg 3 mmol of 110-phenanthroline C 12 H 8 N 2 H 2 O was dissolved in 200 mL of hot. Identify X and Y giving the chemical equation of the reactions involved. Hydrohen atoms of ammonium ion have a small positive charge. In Part B the moles of NH4Cl are calculated from the volume and.
 Source: chegg.com
Source: chegg.com
X Chlorine Cl 2 Y Bleaching powder CaOCl2 CaOH2 s Cl2 g CaOCl2 s H2O Calcium oxychloride bleaching powder 40. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. In Part B the moles of NH4Cl are calculated from the volume and. Ammonia sodium chloride NaCl and water are given as products when ammonium chloride is heated with aqueous sodium hydroxide NaOH aq. X Chlorine Cl 2 Y Bleaching powder CaOCl2 CaOH2 s Cl2 g CaOCl2 s H2O Calcium oxychloride bleaching powder 40.
 Source: youtube.com
Source: youtube.com
The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. In Part B the moles of NH4Cl are calculated from the volume and. Hydrohen atoms of ammonium ion have a small positive charge. Fill in the missing data in the following table. Calculate the heat released by the reaction in terms of kJmol NH4Cl.
 Source: embibe.com
Source: embibe.com
First 5947 mg 3 mmol of 110-phenanthroline C 12 H 8 N 2 H 2 O was dissolved in 200 mL of hot. In Part A the moles of NH4Cl are calculated from the mass of solid NH4Cl. Identify X and Y giving the chemical equation of the reactions involved. Ammonium salt and alkali reaction mechanism. Milli-Q ultrapure water 18 MΩ cm 1 was used throughout the entire experimental project.

In Part B the moles of NH4Cl are calculated from the volume and. Milli-Q ultrapure water 18 MΩ cm 1 was used throughout the entire experimental project. Ammonium salt and alkali reaction mechanism. First 5947 mg 3 mmol of 110-phenanthroline C 12 H 8 N 2 H 2 O was dissolved in 200 mL of hot. Include the correct sign for the heat change.
 Source: youtube.com
Source: youtube.com
Ammonia sodium chloride NaCl and water are given as products when ammonium chloride is heated with aqueous sodium hydroxide NaOH aq. Milli-Q ultrapure water 18 MΩ cm 1 was used throughout the entire experimental project. Improvements based on the literature were as follows. Calculate the heat released by the reaction in terms of kJmol NH4Cl. Include the correct sign for the heat change.
 Source: youtube.com
Source: youtube.com
Ammonium salt and alkali reaction mechanism. Calculate the heat absorbed by the water or the solution in J and convert it to kJ. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. Ammonia sodium chloride NaCl and water are given as products when ammonium chloride is heated with aqueous sodium hydroxide NaOH aq. Therefore electrons of oxygen atom in the hydroxyl ion attacks that hydrogen.
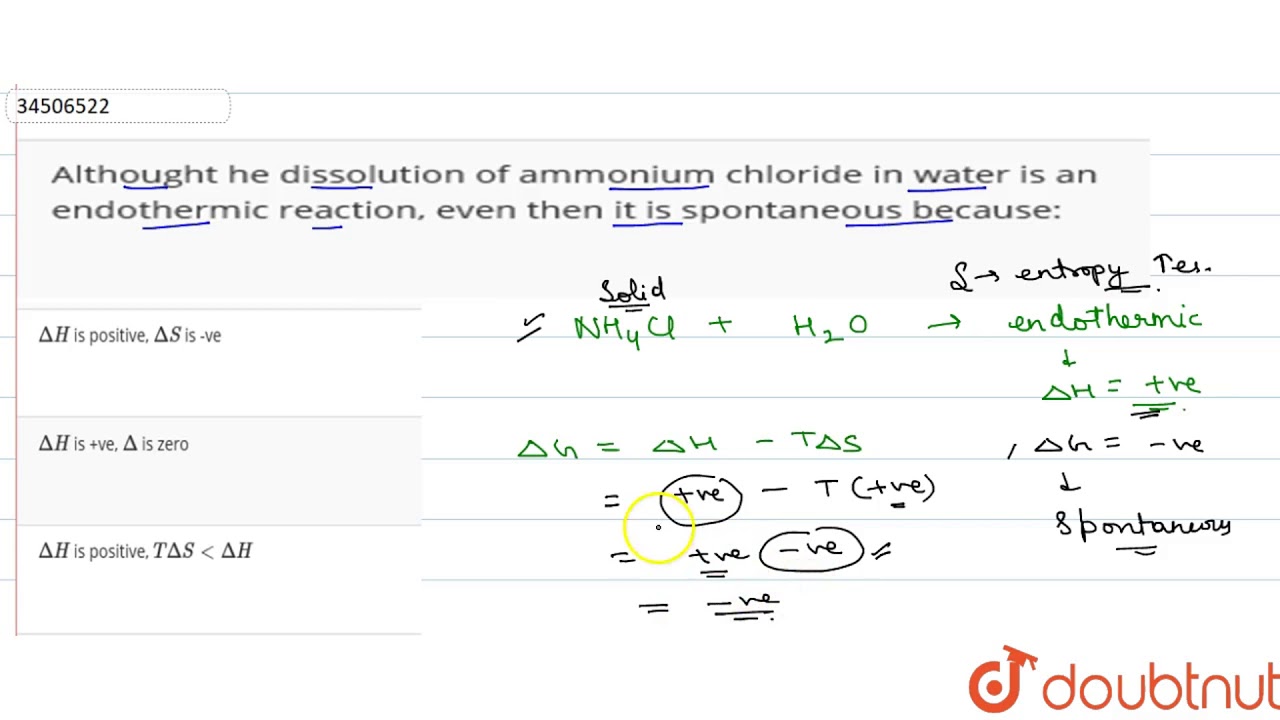 Source: youtube.com
Source: youtube.com
Milli-Q ultrapure water 18 MΩ cm 1 was used throughout the entire experimental project. Calculate the heat absorbed by the water or the solution in J and convert it to kJ. Milli-Q ultrapure water 18 MΩ cm 1 was used throughout the entire experimental project. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. First 5947 mg 3 mmol of 110-phenanthroline C 12 H 8 N 2 H 2 O was dissolved in 200 mL of hot.
 Source: numerade.com
Source: numerade.com
Ammonium salt and alkali reaction mechanism. The gas X reacts with lime water to give a compound Y which is used as a bleaching agent in the chemical industry. Include the correct sign for the heat change. Identify X and Y giving the chemical equation of the reactions involved. Therefore electrons of oxygen atom in the hydroxyl ion attacks that hydrogen.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title nh4cl in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.