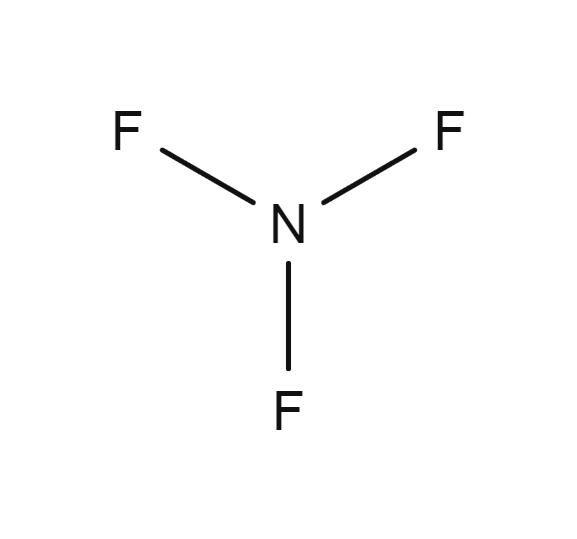
Nitric oxide molar mass
Nitric Oxide Molar Mass. HNO 3 KOH KNO 3 H 2 O 5 The reaction of copper II oxide with hydrogen to form copper metal and water. Nitric acid is a very strong acid turns blue litmus red. Molar Mass of Frequently Calculated Chemicals. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
 Nitric Oxide Wikipedia From en.wikipedia.org
Nitric Oxide Wikipedia From en.wikipedia.org
It has a role as a protic solvent and a reagent. Chemical Properties of Nitric Acid HNO 3. This is the reason why it becomes brownish over time though fresh nitric acid is colourless. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Molecular Weight Molar Mass. Molar Mass of Frequently Calculated Chemicals.
Nitric acid decomposes on standing to form brown nitrogen dioxide.
To update your cookie settings please visit the Cookie Preference Center for this site. HNO 3 KOH KNO 3 H 2 O 5 The reaction of copper II oxide with hydrogen to form copper metal and water. G NH3 mols NH3 x molar mass NH3. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Nitric acid is a nitrogen oxoacid of formula HNO3 in which the nitrogen atom is bonded to a hydroxy group and by equivalent bonds to the remaining two oxygen atoms. N2g 3H2g à 2NH3g o a.
 Source: britannica.com
Source: britannica.com
Tetrahydrobiopterin BH 4 THB also known as sapropterin INN is a cofactor of the three aromatic amino acid hydroxylase enzymes used in the degradation of amino acid phenylalanine and in the biosynthesis of the neurotransmitters serotonin 5-hydroxytryptamine 5-HT melatonin dopamine norepinephrine noradrenaline epinephrine adrenaline and is a cofactor for the production of. Fe2O3s 2 Al s - 2 Fel Al2O3 s What masses of iron III oxide. Its boiling point is 47 C and sublimes slightly above room temperature yielding a colorless gas. Ammonia is produced by the reaction of hydrogen and nitrogen. This is the reason why it becomes brownish over time though fresh nitric acid is colourless.
 Source: diabetesselfmanagement.com
Source: diabetesselfmanagement.com
Electrocatalytic reductions of nitrite nitric oxide and nitrous oxide by thermophilic cytochrome P450 CYP119 in film-modified electrodes and an analytical comparison of its. This is the reason why it becomes brownish over time though fresh nitric acid is colourless. Its boiling point is 47 C and sublimes slightly above room temperature yielding a colorless gas. Nitric acid decomposes on standing to form brown nitrogen dioxide. Electrocatalytic reductions of nitrite nitric oxide and nitrous oxide by thermophilic cytochrome P450 CYP119 in film-modified electrodes and an analytical comparison of its.
 Source: sciencedirect.com
Source: sciencedirect.com
Dinitrogen pentoxide is the chemical compound with the formula N 2 O 5 also known as nitrogen pentoxide or nitric anhydrideIt is one of the binary nitrogen oxides a family of compounds that only contain nitrogen and oxygenIt exists as colourless crystals that melt at 41 C. It is a conjugate acid of a nitrate. Tetrahydrobiopterin BH 4 THB also known as sapropterin INN is a cofactor of the three aromatic amino acid hydroxylase enzymes used in the degradation of amino acid phenylalanine and in the biosynthesis of the neurotransmitters serotonin 5-hydroxytryptamine 5-HT melatonin dopamine norepinephrine noradrenaline epinephrine adrenaline and is a cofactor for the production of. Chemical Properties of Nitric Acid HNO 3. To update your cookie settings please visit the Cookie Preference Center for this site.
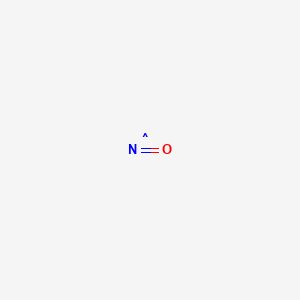
It is a conjugate acid of a nitrate. It is a conjugate acid of a nitrate. 4 The reaction of nitric acid with potassium hydroxide. This is illustrated in the mass spectrum of a bovine serum albumin BSA tryptic digest in Figure 56 in the Chapter 5. Molar Mass of Frequently Calculated Chemicals.
 Source: jbc.org
Source: jbc.org
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. How many moles of N2 reacted if 060 mol of NH3 is. Ammonia is produced by the reaction of hydrogen and nitrogen. Molar Mass of Frequently Calculated Chemicals. CuO H 2 Cu H 2 O 6 The reaction of iron metal with oxygen to form iron III oxide.
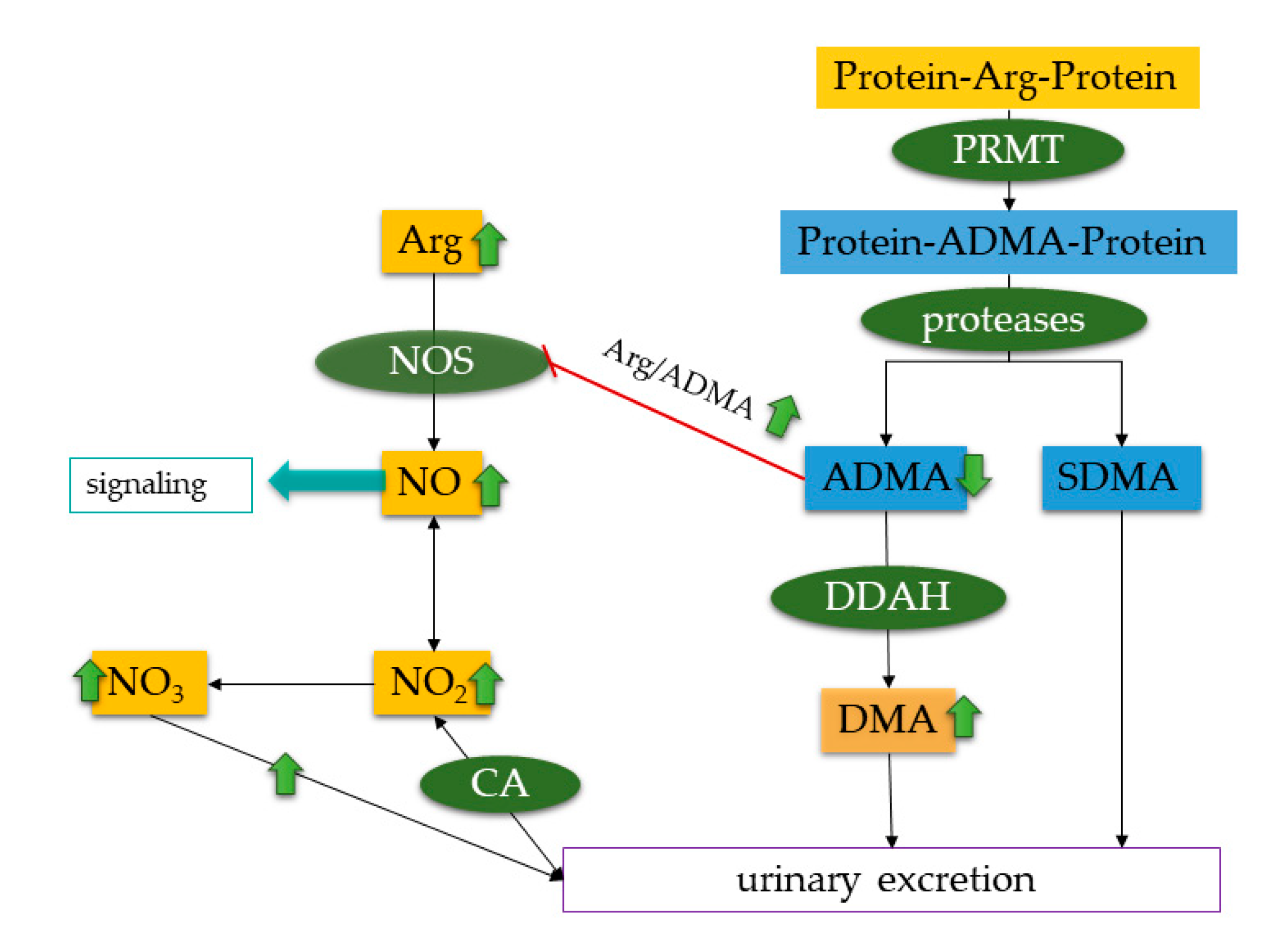 Source: mdpi.com
Source: mdpi.com
Chemical Properties of Nitric Acid HNO 3. Nitric acid is a very strong acid turns blue litmus red. Tetrahydrobiopterin BH 4 THB also known as sapropterin INN is a cofactor of the three aromatic amino acid hydroxylase enzymes used in the degradation of amino acid phenylalanine and in the biosynthesis of the neurotransmitters serotonin 5-hydroxytryptamine 5-HT melatonin dopamine norepinephrine noradrenaline epinephrine adrenaline and is a cofactor for the production of. Dinitrogen pentoxide is the chemical compound with the formula N 2 O 5 also known as nitrogen pentoxide or nitric anhydrideIt is one of the binary nitrogen oxides a family of compounds that only contain nitrogen and oxygenIt exists as colourless crystals that melt at 41 C. 4 Fe 3 O 2 2 Fe 2 O 3 7 The complete combustion of 22-dimethylpropane C 4.

It has a role as a protic solvent and a reagent. This is illustrated in the mass spectrum of a bovine serum albumin BSA tryptic digest in Figure 56 in the Chapter 5. Dinitrogen pentoxide is the chemical compound with the formula N 2 O 5 also known as nitrogen pentoxide or nitric anhydrideIt is one of the binary nitrogen oxides a family of compounds that only contain nitrogen and oxygenIt exists as colourless crystals that melt at 41 C. It has a role as a protic solvent and a reagent. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It is a conjugate acid of a nitrate. Chemical Properties of Nitric Acid HNO 3. Molar Mass of Frequently Calculated Chemicals. Its boiling point is 47 C and sublimes slightly above room temperature yielding a colorless gas. Electrocatalytic reductions of nitrite nitric oxide and nitrous oxide by thermophilic cytochrome P450 CYP119 in film-modified electrodes and an analytical comparison of its.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Although each peptide is present in the same molar abundances there is high variability in the ion abundances with some peptides being completely absent. Tetrahydrobiopterin BH 4 THB also known as sapropterin INN is a cofactor of the three aromatic amino acid hydroxylase enzymes used in the degradation of amino acid phenylalanine and in the biosynthesis of the neurotransmitters serotonin 5-hydroxytryptamine 5-HT melatonin dopamine norepinephrine noradrenaline epinephrine adrenaline and is a cofactor for the production of. How many moles of N2 reacted if 060 mol of NH3 is. 4 The reaction of nitric acid with potassium hydroxide. The purification method is an important factor to consider.
This is illustrated in the mass spectrum of a bovine serum albumin BSA tryptic digest in Figure 56 in the Chapter 5. How many moles of H2 are needed to react with 10 mol of N2. We use cookies to help provide and enhance our service and tailor content. The purification method is an important factor to consider. 4 Fe 3 O 2 2 Fe 2 O 3 7 The complete combustion of 22-dimethylpropane C 4.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title nitric oxide molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.