Nitroglycerin enthalpy of vaporization
Nitroglycerin Enthalpy Of Vaporization. In vehicles the engine that expels the propellant is called a reaction engineAlthough technically a propellant is the reaction mass used to create thrust the term. When both of these conditions are met the reaction occurs naturally. UNK the. 194 103 J.
Nitroglycerin From webbook.nist.gov
A aa aaa aaaa aaacn aaah aaai aaas aab aabb aac aacc aace aachen aacom aacs aacsb aad aadvantage aae aaf aafp aag aah aai aaj aal aalborg aalib aaliyah aall aalto aam. UNK the. In vehicles the engine that expels the propellant is called a reaction engineAlthough technically a propellant is the reaction mass used to create thrust the term. The reported values are expressed in both the liquid L and gas G test. Enthalpies of Combustion. Solucionario quimica de raymond chang 12 edicion.
194 103 J.
Reactions are favorable when they result in a decrease in enthalpy and an increase in entropy of the system. 194 103 J. A propellant or propellent is a mass that is expelled or expanded in such a way as to create a thrust or other motive force in accordance with Newtons third law of motion and propel a vehicle projectile or fluid payload. The effective energy developed by an explosive is always less than the assumed thermodynamic energy. Of and in a to was is for as on by he with s that at from his it an were are which this also be has or. When both of these conditions are met the reaction occurs naturally.
Source: webbook.nist.gov
The effective energy developed by an explosive is always less than the assumed thermodynamic energy. Solucionario quimica de raymond chang 12 edicion. A propellant or propellent is a mass that is expelled or expanded in such a way as to create a thrust or other motive force in accordance with Newtons third law of motion and propel a vehicle projectile or fluid payload. 194 103 J. McMurry - Free ebook download as PDF File pdf Text File txt or read book online for free.
 Source: sciencedirect.com
Source: sciencedirect.com
UNK the. A propellant or propellent is a mass that is expelled or expanded in such a way as to create a thrust or other motive force in accordance with Newtons third law of motion and propel a vehicle projectile or fluid payload. The Heat of Products of Detonation is the energy release at the Chapman-Jouguet C-J condition and refers to the change in enthalpy and is always a negative value. When both of these conditions are met the reaction occurs naturally. The reported values are expressed in both the liquid L and gas G test.
 Source: sciencedirect.com
Source: sciencedirect.com
Complete Solutions Manual General Chemistry Ninth Edition. In vehicles the engine that expels the propellant is called a reaction engineAlthough technically a propellant is the reaction mass used to create thrust the term. The amount of energy for melting solids. The explosion of nitroglycerin releases large volumes of gases and is very exothermic. Reactions are favorable when they result in a decrease in enthalpy and an increase in entropy of the system.
 Source: researchgate.net
Source: researchgate.net
The reported values are expressed in both the liquid L and gas G test. Uploaded from Google Docs. Had first one their its new after but who not they have. Solucionario quimica de raymond chang 12 edicion. UNK the.
Source: webbook.nist.gov
The amount of energy for melting solids. The amount of energy for melting solids. The explosion of nitroglycerin releases large volumes of gases and is very exothermic. Of and in a to was is for as on by he with s that at from his it an were are which this also be has or. The Heat of Products of Detonation is the energy release at the Chapman-Jouguet C-J condition and refers to the change in enthalpy and is always a negative value.
 Source: youtube.com
Source: youtube.com
205 105 J b. The effective energy developed by an explosive is always less than the assumed thermodynamic energy. The amount of energy needed to go from liquid to gas. A propellant or propellent is a mass that is expelled or expanded in such a way as to create a thrust or other motive force in accordance with Newtons third law of motion and propel a vehicle projectile or fluid payload. Solucionario quimica de raymond chang 12 edicion.
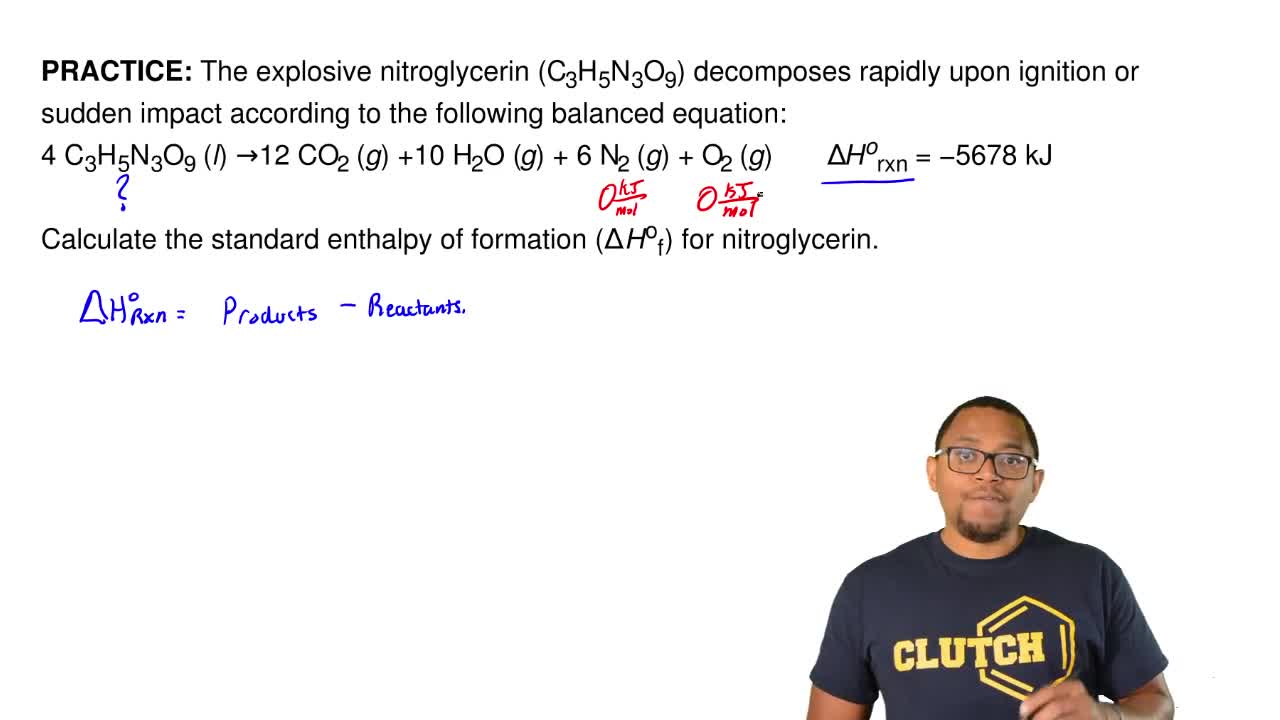 Source: clutchprep.com
Source: clutchprep.com
Solution Manual - Chemistry-4th Ed. When both of these conditions are met the reaction occurs naturally. Had first one their its new after but who not they have. Solution Manual - Chemistry-4th Ed. A propellant or propellent is a mass that is expelled or expanded in such a way as to create a thrust or other motive force in accordance with Newtons third law of motion and propel a vehicle projectile or fluid payload.
 Source: sciencedirect.com
Source: sciencedirect.com
The amount of energy for combusting a substance in oxygen. The amount of energy for melting solids. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. Academiaedu is a platform for academics to share research papers. Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made.
 Source: sciencedirect.com
Source: sciencedirect.com
The effective energy developed by an explosive is always less than the assumed thermodynamic energy. The amount of energy needed to go from liquid to gas. Free essays homework help flashcards research papers book reports term papers history science politics. McMurry - Free ebook download as PDF File pdf Text File txt or read book online for free. Complete Solutions Manual General Chemistry Ninth Edition.
Source: webbook.nist.gov
Free essays homework help flashcards research papers book reports term papers history science politics. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. Uploaded from Google Docs. The effective energy developed by an explosive is always less than the assumed thermodynamic energy. The explosion of nitroglycerin releases large volumes of gases and is very exothermic.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title nitroglycerin enthalpy of vaporization by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.