Nonaromatic nitrogen six membered ring
Nonaromatic Nitrogen Six Membered Ring. Table 5 and Fig. Annulenes completely conjugated monocyclic hydrocarbons may be aromatic nonaromatic or antiaromatic. Inorganic six-membered-ring compounds analogous to benzene have been synthesized. The classic example benzene has a system of six π.
 Heterocyclic Compound Five And Six Membered Rings With Two Or More Heteroatoms Britannica From britannica.com
Heterocyclic Compound Five And Six Membered Rings With Two Or More Heteroatoms Britannica From britannica.com
Indole is a. Table 5 and Fig. In the last post we introduced the concept of aromaticity a property of some unusually stable organic molecules such as benzeneAlthough some aromatic molecules are indeed fragrant hello vanillin. So this strange. In this case the first 2 points to ring connection 1 connecting to nitrogen and the second 2 points to the second connection to the carbon on the right that is to ring connction 2 again a nitrogen. The antiboding MOs would each be paired with a BMO in terms of symmetry about the nonbonding level.
The only exception to this is when we have the excellent leaving group gaseous nitrogen.
3 depict variation of ring strain energy E rs kJ mol-1 with respect to ring sizes 38 of cycloalkanes plot 1 and cyclic sulfides plot 2 determined thermochemically and theoretically DFT derived values for. Identical lead compounds are discovered in a traditional high-throughput screen and structure-based virtual high-throughput screen. Annulenes completely conjugated monocyclic hydrocarbons may be aromatic nonaromatic or antiaromatic. If these two carbons were sp-hybridized as judged from the triple bond and steric number it wouldve probably been impossible since the sp orbitals are pointing in two liner directions. You should anticipate by now that with six carbon atoms there would be six pi MOs three of which would be bonding and three antibonding. The 4 Key Factors.
 Source: britannica.com
Source: britannica.com
Cyclic compounds can be partly or completely conjugated. Therefore the molecule has only 6 electrons in the pi system and is in fact aromatic. Jmol SMILES and SMARTS. 3 depict variation of ring strain energy E rs kJ mol-1 with respect to ring sizes 38 of cycloalkanes plot 1 and cyclic sulfides plot 2 determined thermochemically and theoretically DFT derived values for. The number of nodes would increase in the sequence 012345 in order of the increasing energies of the MOs.
 Source: sciencedirect.com
Source: sciencedirect.com
The number of nodes would increase in the sequence 012345 in order of the increasing energies of the MOs. The 4 Key Factors. You should anticipate by now that with six carbon atoms there would be six pi MOs three of which would be bonding and three antibonding. The only exception to this is when we have the excellent leaving group gaseous nitrogen. The antiboding MOs would each be paired with a BMO in terms of symmetry about the nonbonding level.
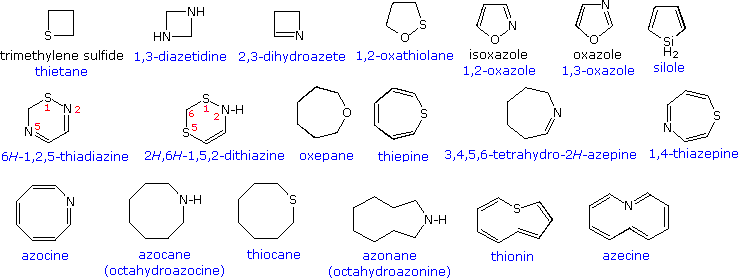 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
The tautomerism of 8-hydroxypyrido23-bpyrazines was investigated by Mason 57JCS4874 using an IR method and it was concluded that the compounds existed in the -one form cf. The pyrylium ion is a six-membered ring that like benzene has three pi bonds. I X-ray crystal structures of 1 and 18 bound to the ATP-binding site of the TβR-I kinase domain discovered using traditional high-throughput screeningCompound 1 shown as the thinner wire-frame is the original hit from the HTS and is identical to that which was. Indole is a. In this case the first 2 points to ring connection 1 connecting to nitrogen and the second 2 points to the second connection to the carbon on the right that is to ring connction 2 again a nitrogen.
 Source: britannica.com
Source: britannica.com
It just looks bothering to have a triple bond in a six-membered ring where the angles are 120 o. So this strange. Table 5 and Fig. I X-ray crystal structures of 1 and 18 bound to the ATP-binding site of the TβR-I kinase domain discovered using traditional high-throughput screeningCompound 1 shown as the thinner wire-frame is the original hit from the HTS and is identical to that which was. The number of nodes would increase in the sequence 012345 in order of the increasing energies of the MOs.
 Source: britannica.com
Source: britannica.com
For example borazine is a six-membered ring composed of alternating boron and nitrogen atoms each with one hydrogen attached. It just looks bothering to have a triple bond in a six-membered ring where the angles are 120 o. Inorganic six-membered-ring compounds analogous to benzene have been synthesized. You should anticipate by now that with six carbon atoms there would be six pi MOs three of which would be bonding and three antibonding. It has a delocalized π system and undergoes.
 Source: britannica.com
Source: britannica.com
The only exception to this is when we have the excellent leaving group gaseous nitrogen. The tautomerism of 8-hydroxypyrido23-bpyrazines was investigated by Mason 57JCS4874 using an IR method and it was concluded that the compounds existed in the -one form cf. The classic example benzene has a system of six π. So this strange. In this case the first 2 points to ring connection 1 connecting to nitrogen and the second 2 points to the second connection to the carbon on the right that is to ring connction 2 again a nitrogen.
 Source: britannica.com
Source: britannica.com
The classic example benzene has a system of six π. Rules For Aromaticity. In this case the first 2 points to ring connection 1 connecting to nitrogen and the second 2 points to the second connection to the carbon on the right that is to ring connction 2 again a nitrogen. I X-ray crystal structures of 1 and 18 bound to the ATP-binding site of the TβR-I kinase domain discovered using traditional high-throughput screeningCompound 1 shown as the thinner wire-frame is the original hit from the HTS and is identical to that which was. Annulenes completely conjugated monocyclic hydrocarbons may be aromatic nonaromatic or antiaromatic.
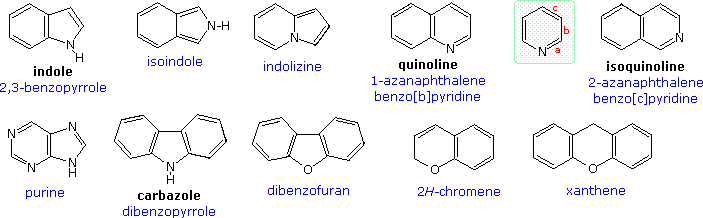 Source: www2.chemistry.msu.edu
Source: www2.chemistry.msu.edu
The classic example benzene has a system of six π. If these two carbons were sp-hybridized as judged from the triple bond and steric number it wouldve probably been impossible since the sp orbitals are pointing in two liner directions. Inorganic six-membered-ring compounds analogous to benzene have been synthesized. The tautomerism of 8-hydroxypyrido23-bpyrazines was investigated by Mason 57JCS4874 using an IR method and it was concluded that the compounds existed in the -one form cf. 71CRC2731529The tautomerism of some deaza analogues of riboflavin has been studied 77TL2551.
 Source: cheminfographic.wordpress.com
Source: cheminfographic.wordpress.com
Annulenes completely conjugated monocyclic hydrocarbons may be aromatic nonaromatic or antiaromatic. Table 5 and Fig. 3 depict variation of ring strain energy E rs kJ mol-1 with respect to ring sizes 38 of cycloalkanes plot 1 and cyclic sulfides plot 2 determined thermochemically and theoretically DFT derived values for. Ring strain energy of sulphur containing eight membered ring is similar to that of the 8-membered cycloalkane system as demonstrated in a theoretical study. You should anticipate by now that with six carbon atoms there would be six pi MOs three of which would be bonding and three antibonding.
 Source: pdfprof.com
Source: pdfprof.com
The pyrylium ion is a six-membered ring that like benzene has three pi bonds. The tautomerism of 8-hydroxypyrido23-bpyrazines was investigated by Mason 57JCS4874 using an IR method and it was concluded that the compounds existed in the -one form cf. Compounds that have a monocyclic planar conjugated system containing 4n 2 π-electrons for whole numbers n are aromatic and exhibit an unusual stability. 71CRC2731529The tautomerism of some deaza analogues of riboflavin has been studied 77TL2551. In the last post we introduced the concept of aromaticity a property of some unusually stable organic molecules such as benzeneAlthough some aromatic molecules are indeed fragrant hello vanillin.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title nonaromatic nitrogen six membered ring by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.