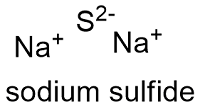Oxide of osmium
Oxide Of Osmium. The Upjohn Dihydroxylation allows the syn-selective preparation of 12-diols from alkenes by the use of osmium tetroxide as a catalyst and a stoichiometric amount of an oxidant such as NMO N-methyl morpholine-N-Oxide. It has a role as an. The alkali metal oxides M 2 O M Li Na K Rb crystallise in the antifluorite structure. Osmium tetroxide is an osmium coordination entity consisting of four oxygen atoms bound to a central osmium atom via covalent double bonds.
 Osmium Viii Oxide From heraeus.com
Osmium Viii Oxide From heraeus.com
It is used in ceramics and glasses. Once ignited magnesium metal burns in air with a characteristic blinding bright white flame to give a mixture of white magnesium oxide MgO and magnesium nitride Mg 3 N 2. In this motif the positions of the anions and cations are reversed. The Upjohn Dihydroxylation allows the syn-selective preparation of 12-diols from alkenes by the use of osmium tetroxide as a catalyst and a stoichiometric amount of an oxidant such as NMO N-methyl morpholine-N-Oxide. It has a characteristic acrid chlorine-like odorThe element name osmium is derived from osme Greek for odorOsO 4 is volatile. The main target users are workers and those responsible for occupational safety and health.
Once ignited magnesium metal burns in air with a characteristic blinding bright white flame to give a mixture of white magnesium oxide MgO and magnesium nitride Mg 3 N 2.
It is used in ceramics and glasses. The main target users are workers and those responsible for occupational safety and health. In this motif the positions of the anions and cations are reversed. It has a role as an. The compound is the base anhydride of sodium hydroxide. The surface of magnesium metal is covered with a thin layer of oxide that helps protect the metal from attack by air.
 Source: chemistryworld.com
Source: chemistryworld.com
Na 2 O H 2 O 2 NaOH. The main target users are workers and those responsible for occupational safety and health. The alkali metal oxides M 2 O M Li Na K Rb crystallise in the antifluorite structure. In a blatant plug for the Reagent Guide each Friday I profile a different reagent that is commonly encountered in Org 1 Org 2. When water is added to sodium oxide NaOH is produced.
 Source: bioprocessonline.com
Source: bioprocessonline.com
Magnesium is a silvery white metal. The compound is the base anhydride of sodium hydroxide. Once ignited magnesium metal burns in air with a characteristic blinding bright white flame to give a mixture of white magnesium oxide MgO and magnesium nitride Mg 3 N 2. The ICSC project is a common undertaking between the World Health Organization WHO and. Osmium tetroxide is an osmium coordination entity consisting of four oxygen atoms bound to a central osmium atom via covalent double bonds.
 Source: americanelements.com
Source: americanelements.com
Once ignited magnesium metal burns in air with a characteristic blinding bright white flame to give a mixture of white magnesium oxide MgO and magnesium nitride Mg 3 N 2. OsO4 Osmium Tetroxide As A Reagent For the Dihydroxylation Of Alkenes. OsmiumVIII oxide forms monoclinic crystals. The main target users are workers and those responsible for occupational safety and health. When water is added to sodium oxide NaOH is produced.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It is also moderately soluble in water with which it reacts reversibly to form osmic acid see below. Oxides result when elements are oxidized by oxygen in air. Once ignited magnesium metal burns in air with a characteristic blinding bright white flame to give a mixture of white magnesium oxide MgO and magnesium nitride Mg 3 N 2. Todays reagent is among one of the best and most useful at what it. The surface of magnesium metal is covered with a thin layer of oxide that helps protect the metal from attack by air.
 Source: nanochemazone.com
Source: nanochemazone.com
The Upjohn Dihydroxylation allows the syn-selective preparation of 12-diols from alkenes by the use of osmium tetroxide as a catalyst and a stoichiometric amount of an oxidant such as NMO N-methyl morpholine-N-Oxide. Todays reagent is among one of the best and most useful at what it. In a blatant plug for the Reagent Guide each Friday I profile a different reagent that is commonly encountered in Org 1 Org 2. The alkali metal oxides M 2 O M Li Na K Rb crystallise in the antifluorite structure. In this motif the positions of the anions and cations are reversed.
 Source: heraeus.com
Source: heraeus.com
It sublimes at room temperatureIt is soluble in a wide range of organic solvents. Most of the Earths crust consists of oxides. It has a characteristic acrid chlorine-like odorThe element name osmium is derived from osme Greek for odorOsO 4 is volatile. OsmiumVIII oxide forms monoclinic crystals. It has a role as an.
 Source: chm.bris.ac.uk
Source: chm.bris.ac.uk
When water is added to sodium oxide NaOH is produced. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. Magnesium is a silvery white metal. The compound is the base anhydride of sodium hydroxide. It sublimes at room temperatureIt is soluble in a wide range of organic solvents.
 Source: periodictable.ru
Source: periodictable.ru
Todays reagent is among one of the best and most useful at what it. Na 2 O H 2 O 2 NaOH. Most of the Earths crust consists of oxides. Osmium tetroxide is an osmium coordination entity consisting of four oxygen atoms bound to a central osmium atom via covalent double bonds. The Upjohn Dihydroxylation allows the syn-selective preparation of 12-diols from alkenes by the use of osmium tetroxide as a catalyst and a stoichiometric amount of an oxidant such as NMO N-methyl morpholine-N-Oxide.
 Source: glentham.com
Source: glentham.com
Osmium tetroxide is an osmium coordination entity consisting of four oxygen atoms bound to a central osmium atom via covalent double bonds. Magnesium is a silvery white metal. Oxides result when elements are oxidized by oxygen in air. Sodium oxide is a chemical compound with the formula Na 2 O. It has a role as an.
 Source: indiamart.com
Source: indiamart.com
Oxides result when elements are oxidized by oxygen in air. When water is added to sodium oxide NaOH is produced. In this motif the positions of the anions and cations are reversed. It has a role as an. The main target users are workers and those responsible for occupational safety and health.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title oxide of osmium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.