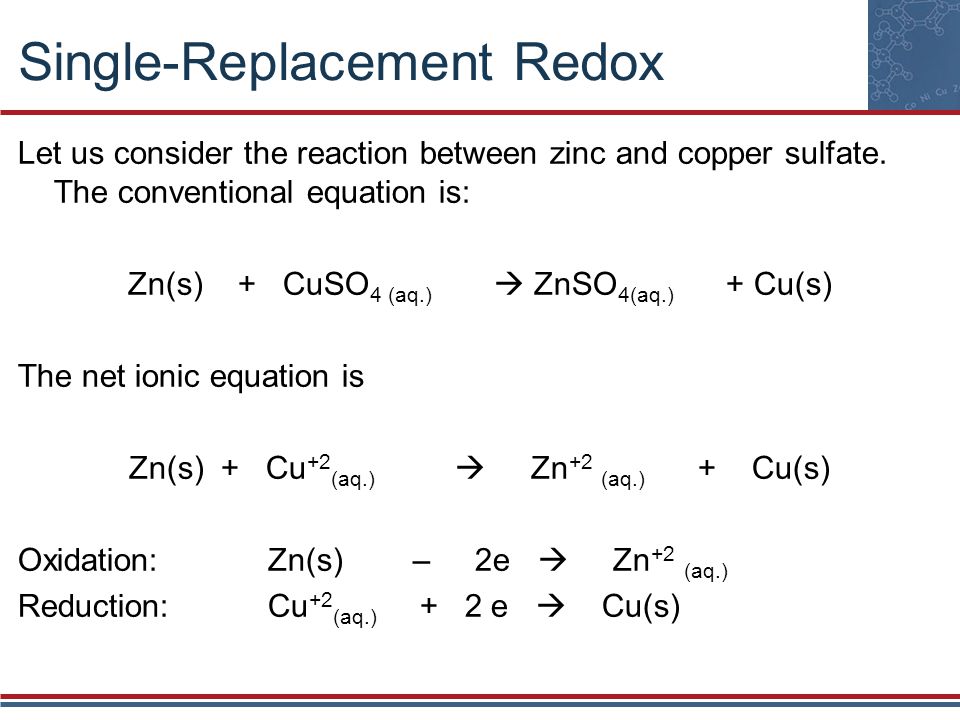
Pbcro4 molar mass
Pbcro4 Molar Mass. Q24 Polychlorinated biphenyls PCBs. Determine the mass of glucose molar mass 180 gmol needed to add to 5000 g of water to change the vapor pressure to 231 torr. For C17H25N the molar mass 17C 25H 1N equals 24343 gmole and the three theoretical values for by weight are calculated as follows. Academiaedu is a platform for academics to share research papers.
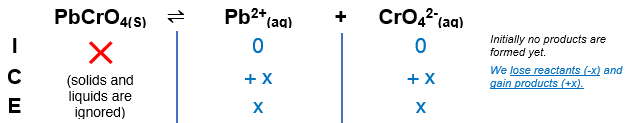 Calculate The Solubility At 25 Celsius Of Clutch Prep From clutchprep.com
Calculate The Solubility At 25 Celsius Of Clutch Prep From clutchprep.com
Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. Academiaedu is a platform for academics to share research papers. Its molar mass is 102 gmol. The vapor pressure of pure water at this temperature is 238 torr. Determine the vapor pressure of the solution. These data are consistent with the experimental values cited in the problem.
Q24 Polychlorinated biphenyls PCBs.
150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C. James Holler Stanley R. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Analytical Chemistry Douglas A. Molar Mass of Frequently Calculated Chemicals. At 40 C heptane has a vapor pressure of 920.
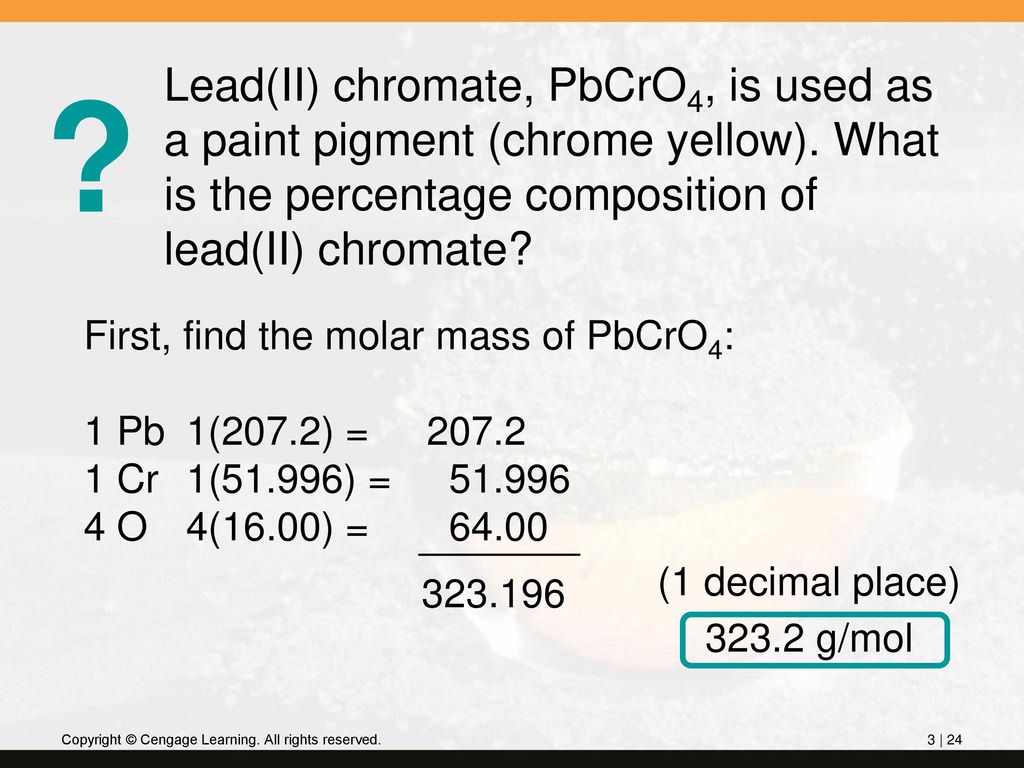 Source: slideplayer.com
Source: slideplayer.com
Analytical Chemistry Douglas A. 150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C. The vapor pressure of pure water at this temperature is 238 torr. James Holler Stanley R. Complete Solutions Manual General Chemistry Ninth Edition.
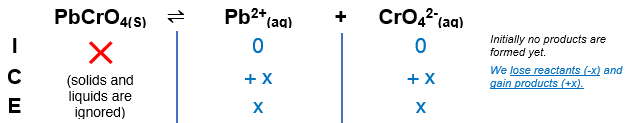 Source: clutchprep.com
Source: clutchprep.com
At 40 C heptane has a vapor pressure of 920. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N. Molar Mass of Frequently Calculated Chemicals. Determine the molecular formula of cadaverine. James Holler Stanley R.
 Source: slideplayer.com
Source: slideplayer.com
150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C. Academiaedu is a platform for academics to share research papers. Complete Solutions Manual General Chemistry Ninth Edition. These data are consistent with the experimental values cited in the problem. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N.

Analytical Chemistry Douglas A. Its molar mass is 102 gmol. These data are consistent with the experimental values cited in the problem. For C17H25N the molar mass 17C 25H 1N equals 24343 gmole and the three theoretical values for by weight are calculated as follows. The vapor pressure of pure water at this temperature is 238 torr.
 Source: sciencedirect.com
Source: sciencedirect.com
Analytical Chemistry Douglas A. James Holler Stanley R. Determine the molecular formula of cadaverine. Academiaedu is a platform for academics to share research papers. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N.
At 40 C heptane has a vapor pressure of 920. James Holler Stanley R. 1 mol X mol X 00549 mol NaOH x 00274 mol X 2 mol NaOH 500 g X molar mass 182 gmol 00274 mol Because the molar mass is twice the empirical formula mass the molecular formula is. Its molar mass is 102 gmol. These data are consistent with the experimental values cited in the problem.
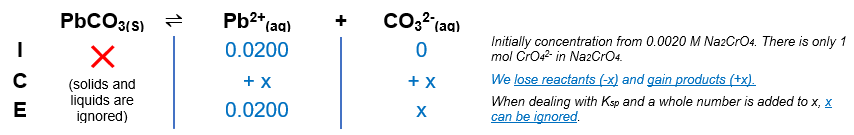 Source: clutchprep.com
Source: clutchprep.com
Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. 1 mol X mol X 00549 mol NaOH x 00274 mol X 2 mol NaOH 500 g X molar mass 182 gmol 00274 mol Because the molar mass is twice the empirical formula mass the molecular formula is. Academiaedu is a platform for academics to share research papers.
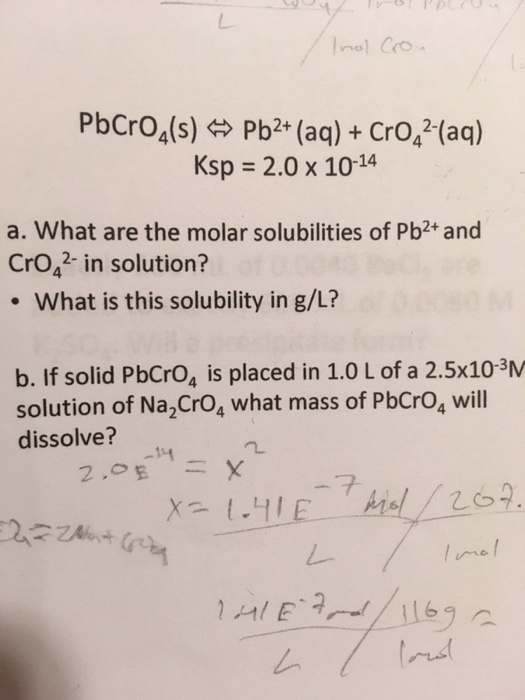
It is 5877 C 1381 H and 2742 N. Determine the mass of glucose molar mass 180 gmol needed to add to 5000 g of water to change the vapor pressure to 231 torr. For C17H25N the molar mass 17C 25H 1N equals 24343 gmole and the three theoretical values for by weight are calculated as follows. Q24 Polychlorinated biphenyls PCBs. Determine the molecular formula of cadaverine.
 Source: quizlet.com
Source: quizlet.com
Q24 Polychlorinated biphenyls PCBs. James Holler Stanley R. Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. Complete Solutions Manual General Chemistry Ninth Edition. 150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C.
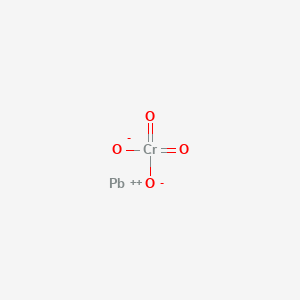
 Source: en.wikipedia.org
Source: en.wikipedia.org
For C20H25N3O the molar mass. Analytical Chemistry Douglas A. Determine the mass of glucose molar mass 180 gmol needed to add to 5000 g of water to change the vapor pressure to 231 torr. C 2042 g C 100 8389 2434 g C17 H 25 N H 2520 g H 100 1035 2434 g C17 H 25 N N 1401 g N 100 576 2434 g C17 H 25 N. The vapor pressure of pure water at this temperature is 238 torr.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title pbcro4 molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.