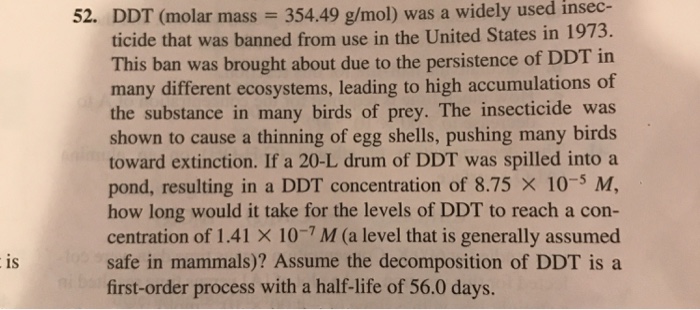
Phosphorus trihydride symbol
Phosphorus Trihydride Symbol. Since Phosphorous is in Period Three on the periodic table it can have more than eight valence electrons. The synthesis of nitrogen trihydride from nitrogen gas and hydrogen gas is shown by which balanced chemical equation. Youll sometimes see this called phosphorus trihydride but that is a less common name. KF potassium fluoride 2.

Write the chemical formula for diphosphorus pentoxide - this compound contains two phosphorus atoms and five oxygen atoms. KF potassium fluoride 2. First quickly scan the worksheet and circle any metals as a symbol or as a name that is a transition metal. A multivalent nonmetal of the nitrogen group phosphorus as a mineral is almost always present in its maximally oxidized state as inorganic phosphate rocks. P 2O 5 2. Phosphorus sulfide P4S7 EINECS 234-868-9.
Phosphorus ˈ f ɒ s f ər ə s fos-fər-əs is the chemical element that has the symbol P and atomic number 15.
Arsane is an inorganic compound with the formula As H 3This flammable pyrophoric and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. One of the most The Lewis structure for this hydrogen ion is H For the main. One of the element name followed by its electrical charge. How many 4p electrons are in Br. Phosphane is a colourless flammable very toxic gas compound with the chemical formula PH 3 classed as a pnictogen hydridePure phosphine is odourless but technical grade samples have a highly unpleasant odour like rotting fish due to the presence of substituted phosphine and diphosphane P 2 H 4With traces of P 2 H 4 present PH 3 is spontaneously flammable in. Use the periodic table to determine each of the following.

How many 4p electrons are in Br. Use the periodic table to determine each of the following. Unit chemical names and formulas review wksh 1 answers. Phosphorus ˈ f ɒ s f ər ə s fos-fər-əs is the chemical element that has the symbol P and atomic number 15. A multivalent nonmetal of the nitrogen group phosphorus as a mineral is almost always present in its maximally oxidized state as inorganic phosphate rocks.
 Source: chemnet.com
Source: chemnet.com
You will now learn how to name compounds and write the formulas for compounds containing nonmetals only. Some elements have more than one oxidation number. Unit chemical names and formulas review wksh 1 answers. N2g 3H2g 2NH3g During a physical science lab investigating chemical reactions several students placed a 30g antacid tablet in a 30g zip-lock bag. Any structure for P4S3 that obeys valency rules Increased nuclear attraction for electrons increased nuclear charge sulfur has more protons in nucleus 1 mark Correctly identify that the forces are stronger between sulfur molecules than between the.
 Source: gograph.com
Source: gograph.com
Any structure for P4S3 that obeys valency rules Increased nuclear attraction for electrons increased nuclear charge sulfur has more protons in nucleus 1 mark Correctly identify that the forces are stronger between sulfur molecules than between the. Lines for pairs of electrons and dots for single electrons are placed around the chemical symbols to represent the valence electrons. N2g 3H2g 2NH3g During a physical science lab investigating chemical reactions several students placed a 30g antacid tablet in a 30g zip-lock bag. How many 2s electrons are in Be. Use the table of ions in Model 1 to answer the following questions.
 Source: en.wikipedia.org
Source: en.wikipedia.org
An -ite or -ate ending means there is a _____ ion that includes oxygen in the formula. C2br2 geometry - afiuniquspl. KF potassium fluoride 2. In the compound zinc phosphide what is the charge on the phosphide ion. Caddell COMPOUND LEWIS STRUCTURE 10.
 Source: webelements.com
Source: webelements.com
How many 3d electrons are in V. An -ite or -ate ending means there is a _____ ion that includes oxygen in the formula. KF potassium fluoride 2. X is the symbol of the element. Phosphorus sulfide P4S7 EINECS 234-868-9.
 Source: youtube.com
Source: youtube.com
One of the most The Lewis structure for this hydrogen ion is H For the main. SO 2 b water. Caddell COMPOUND LEWIS STRUCTURE 10. Phosphane is a colourless flammable very toxic gas compound with the chemical formula PH 3 classed as a pnictogen hydridePure phosphine is odourless but technical grade samples have a highly unpleasant odour like rotting fish due to the presence of substituted phosphine and diphosphane P 2 H 4With traces of P 2 H 4 present PH 3 is spontaneously flammable in. Lines for pairs of electrons and dots for single electrons are placed around the chemical symbols to represent the valence electrons.
 Source: en.wikipedia.org
Source: en.wikipedia.org
How many 3d electrons are in V. Lines for pairs of electrons and dots for single electrons are placed around the chemical symbols to represent the valence electrons. Circle the symbol for the metal in each of the compounds in Model 2. The synthesis of nitrogen trihydride from nitrogen gas and hydrogen gas is shown by which balanced chemical equation. How many 3d electrons are in V.

How many 3d electrons are in V. Now if we have more than one atom shown in a Lewis structure then we will have to show what we think are the bonds holding. Circle the symbol for the metal in each of the compounds in Model 2. Lines for pairs of electrons and dots for single electrons are placed around the chemical symbols to represent the valence electrons. The synthesis of nitrogen trihydride from nitrogen gas and hydrogen gas is shown by which balanced chemical equation.
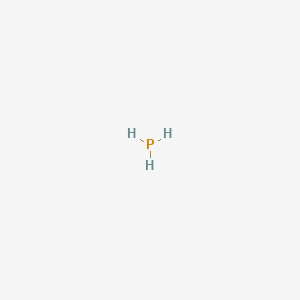
C2br2 geometry - afiuniquspl. Some elements have more than one oxidation number. P 2O 5 2. One of the most The Lewis structure for this hydrogen ion is H For the main. 7 ammonia nitrogen trihydride NH3.
 Source: youtube.com
Source: youtube.com
A multivalent nonmetal of the nitrogen group phosphorus as a mineral is almost always present in its maximally oxidized state as inorganic phosphate rocks. Circle the symbol for the metal in each of the compounds in Model 2. See the front cover of the text which has a listing of the symbols for the various elements and corresponding atomic number or. Represent core electrons with the symbol of the previous noble gas in brackets. Phosphorus ˈ f ɒ s f ər ə s fos-fər-əs is the chemical element that has the symbol P and atomic number 15.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title phosphorus trihydride symbol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.