Physical properties of 1 chlorobutane
Physical Properties Of 1 Chlorobutane. I 2-Chloro-3-methylpentane ii 1-Chloro-4-ethylcydohexane iii 4-tert. NCERT Solutions CBSE Sample Papers Chemistry Class 12 Chemistry NCERT IN TEXT QUESTIONS. The shorter chain less lipid soluble solvents were the more potent at decreasing triglyceride secretion in vivo. Butyl-3-iodoheptane iv 14-Dibromobut-2-ene v 1-Bromo-4-sec.
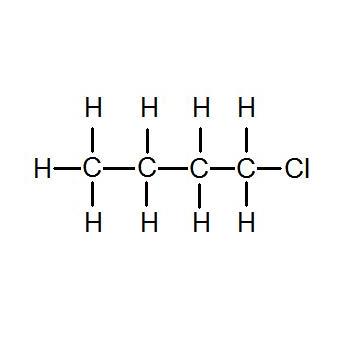
 1 Chlorobutane 109 69 3 C4h9cl Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
1 Chlorobutane 109 69 3 C4h9cl Density Melting Point Boiling Point Structural Formula Synthesis From chemsynthesis.com
Il appartient à la famille des hydrocarbures aromatiques monocycliques car le cycle formé par les six atomes de carbone est plan et comporte six électrons délocalisésDans les conditions usuelles le benzène est un liquide incolore dodeur caractéristique volatil très. We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. Butyl-3-iodoheptane iv 14-Dibromobut-2-ene v 1-Bromo-4-sec. Why is sulphuric acid not used. A π bond being a weaker bond is. McBee and Paul Weber Industrial Engineering Chemistry 1936 28 3 333-339.
The shorter chain less lipid soluble solvents were the more potent at decreasing triglyceride secretion in vivo.
101 Write structures of the following compounds. Il appartient à la famille des hydrocarbures aromatiques monocycliques car le cycle formé par les six atomes de carbone est plan et comporte six électrons délocalisésDans les conditions usuelles le benzène est un liquide incolore dodeur caractéristique volatil très. Chlorination of Paraffins B. Butyl-3-iodoheptane iv 14-Dibromobut-2-ene v 1-Bromo-4-sec. 101 Write structures of the following compounds. Significant dose related decreases in serum triglyceride levels at 2 hours followed the administration of 1-chloropropane 1-chlorobutane 1-chloropentane 1-chlorohexane 12-dichloroethane 13-dichloropropane 14-dichlorobutane and 15-dichloropentane.

The name of an alkyl group is obtained by dropping the suffix -ane of the. McBee and Paul Weber Industrial Engineering Chemistry 1936 28 3 333-339. Check Your Learning Name the following molecule. Why is sulphuric acid not used. Alkenes are much more reactive than alkanes because the latextextCtextClatex moiety is a reactive functional group.
Butane 1-Chlorobutane 1-Bromobutane 1-Iodobutane ii 1-Iodobutane 1-Bromobutane 1-Chlorobutane Butane iii Butane 1-Iodobutane 1-Bromobutane 1-Chlorobutane iv Butane 1-Chlorobutane 1-Iodobutane 1-Bromobutane. NCERT Solutions CBSE Sample Papers Chemistry Class 12 Chemistry NCERT IN TEXT QUESTIONS. These molecular models show the structural and geometric isomers of butene. McBee and Paul Weber Industrial Engineering Chemistry 1936 28 3 333-339. The chlorine at position 1 will be described by adding 1-chloro- resulting in the name of the molecule being 2-bromo-1-chlorobutane.

In freshly prepared. The shorter chain less lipid soluble solvents were the more potent at decreasing triglyceride secretion in vivo. Alkenes are much more reactive than alkanes because the latextextCtextClatex moiety is a reactive functional group. NCERT Solutions CBSE Sample Papers Chemistry Class 12 Chemistry NCERT IN TEXT QUESTIONS. I 2-Chloro-3-methylpentane ii 1-Chloro-4-ethylcydohexane iii 4-tert.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
A π bond being a weaker bond is. I 2-Chloro-3-methylpentane ii 1-Chloro-4-ethylcydohexane iii 4-tert. 101 Write structures of the following compounds. The shorter chain less lipid soluble solvents were the more potent at decreasing triglyceride secretion in vivo. The different geometries produce different physical properties such as boiling point that may make separation of the isomers possible.
 Source: chemsynthesis.com
Source: chemsynthesis.com
One notable advance of Hass work here was in obtaining pure standards for each of the monochlorination products so that their physical properties could be measured and the yields of the reactions accurately reported. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. Significant dose related decreases in serum triglyceride levels at 2 hours followed the administration of 1-chloropropane 1-chlorobutane 1-chloropentane 1-chlorohexane 12-dichloroethane 13-dichloropropane 14-dichlorobutane and 15-dichloropentane. The name of an alkyl group is obtained by dropping the suffix -ane of the. Check Your Learning Name the following molecule.
 Source: chemsrc.com
Source: chemsrc.com
McBee and Paul Weber Industrial Engineering Chemistry 1936 28 3 333-339. The chlorine at position 1 will be described by adding 1-chloro- resulting in the name of the molecule being 2-bromo-1-chlorobutane. One notable advance of Hass work here was in obtaining pure standards for each of the monochlorination products so that their physical properties could be measured and the yields of the reactions accurately reported. These molecular models show the structural and geometric isomers of butene. Hydrogen chloride solution Hydrochloric.

Enantiomers possess identical physical properties. McBee and Paul Weber Industrial Engineering Chemistry 1936 28 3 333-339. NCERT Solutions CBSE Sample Papers Chemistry Class 12 Chemistry NCERT IN TEXT QUESTIONS. We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production.
 Source: en.wikipedia.org
Source: en.wikipedia.org
It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite. These molecular models show the structural and geometric isomers of butene. The shorter chain less lipid soluble solvents were the more potent at decreasing triglyceride secretion in vivo. It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite. Enantiomers possess identical physical properties.
 Source: tcichemicals.com
Source: tcichemicals.com
McBee and Paul Weber Industrial Engineering Chemistry 1936 28 3 333-339. We call a substituent that contains one less hydrogen than the corresponding alkane an alkyl group. A π bond being a weaker bond is. Il appartient à la famille des hydrocarbures aromatiques monocycliques car le cycle formé par les six atomes de carbone est plan et comporte six électrons délocalisésDans les conditions usuelles le benzène est un liquide incolore dodeur caractéristique volatil très. Check Your Learning Name the following molecule.
Analytical 4 ACS reagent 2 BioReagent 2 Technique. The shorter chain less lipid soluble solvents were the more potent at decreasing triglyceride secretion in vivo. A π bond being a weaker bond is. These molecular models show the structural and geometric isomers of butene. Il appartient à la famille des hydrocarbures aromatiques monocycliques car le cycle formé par les six atomes de carbone est plan et comporte six électrons délocalisésDans les conditions usuelles le benzène est un liquide incolore dodeur caractéristique volatil très.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title physical properties of 1 chlorobutane by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.