Polycyclic aromatic hydrocarbon clusters
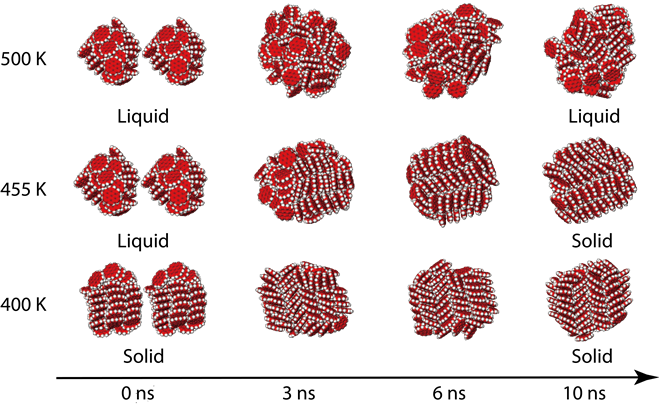
Polycyclic Aromatic Hydrocarbon Clusters. Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. These pyrolyses involve not only cracking to give H 2 but also recondensation. When the temperature is increased from 500 to 900 C most PAHs increase.
 Computational Modelling Group Preprint 131 From como.ceb.cam.ac.uk
Computational Modelling Group Preprint 131 From como.ceb.cam.ac.uk
Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. When the temperature is increased from 500 to 900 C most PAHs increase. Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons. With the increase of the temperature the percentage of light PAHs decrease and the. Products include the clusters pentaborane and decaborane.
Products include the clusters pentaborane and decaborane.
Other examples of aromatic ions include the cyclopropenium cation 2 π-electrons and cyclooctatetraenyl dianion 10 π electrons. When the temperature is increased from 500 to 900 C most PAHs increase. With the increase of the temperature the percentage of light PAHs decrease and the. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. These pyrolyses involve not only cracking to give H 2 but also recondensation. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16.
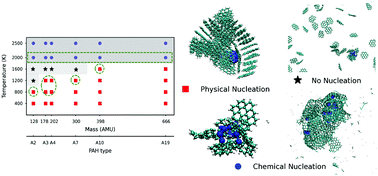
Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. Products include the clusters pentaborane and decaborane. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. These pyrolyses involve not only cracking to give H 2 but also recondensation. With the increase of the temperature the percentage of light PAHs decrease and the.

The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. When the temperature is increased from 500 to 900 C most PAHs increase. With the increase of the temperature the percentage of light PAHs decrease and the. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. Products include the clusters pentaborane and decaborane.
 Source: pnas.org
Source: pnas.org
With the increase of the temperature the percentage of light PAHs decrease and the. With the increase of the temperature the percentage of light PAHs decrease and the. Other examples of aromatic ions include the cyclopropenium cation 2 π-electrons and cyclooctatetraenyl dianion 10 π electrons. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. These pyrolyses involve not only cracking to give H 2 but also recondensation.
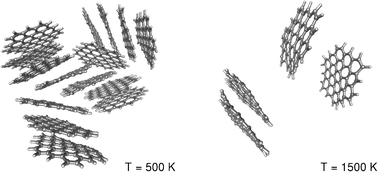
Other examples of aromatic ions include the cyclopropenium cation 2 π-electrons and cyclooctatetraenyl dianion 10 π electrons. These pyrolyses involve not only cracking to give H 2 but also recondensation. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons.
 Source: researchgate.net
Source: researchgate.net
Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons. Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. These pyrolyses involve not only cracking to give H 2 but also recondensation. With the increase of the temperature the percentage of light PAHs decrease and the.
 Source: link.springer.com
Source: link.springer.com
Other examples of aromatic ions include the cyclopropenium cation 2 π-electrons and cyclooctatetraenyl dianion 10 π electrons. Products include the clusters pentaborane and decaborane. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. These pyrolyses involve not only cracking to give H 2 but also recondensation. When the temperature is increased from 500 to 900 C most PAHs increase.
 Source: researchgate.net
Source: researchgate.net
These pyrolyses involve not only cracking to give H 2 but also recondensation. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. When the temperature is increased from 500 to 900 C most PAHs increase. Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons. These pyrolyses involve not only cracking to give H 2 but also recondensation.
 Source: sciencedirect.com
Source: sciencedirect.com
When the temperature is increased from 500 to 900 C most PAHs increase. Products include the clusters pentaborane and decaborane. Naphthalene is the most abundant PAH among all the polycyclic aromatic hydrocarbons. These pyrolyses involve not only cracking to give H 2 but also recondensation. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings.
 Source: como.ceb.cam.ac.uk
Source: como.ceb.cam.ac.uk
With the increase of the temperature the percentage of light PAHs decrease and the. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings. When the temperature is increased from 500 to 900 C most PAHs increase. With the increase of the temperature the percentage of light PAHs decrease and the.
 Source: sciencedirect.com
Source: sciencedirect.com
These pyrolyses involve not only cracking to give H 2 but also recondensation. Products include the clusters pentaborane and decaborane. The cyclopentadienyl anion is formed very easily and thus 13-cyclopentadiene is a very acidic hydrocarbon with a pK a of 16. With the increase of the temperature the percentage of light PAHs decrease and the. Polycyclic aromatic hydrocarbons are molecules containing two or more simple aromatic rings.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title polycyclic aromatic hydrocarbon clusters by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.