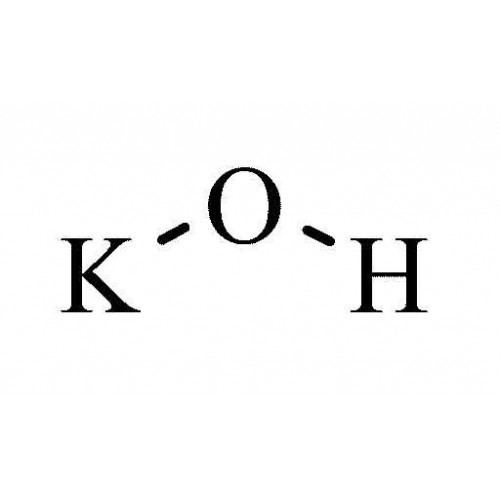
Potassium hydroxide molecules
Potassium Hydroxide Molecules. KOH with K and OH-ions. Additionally oxidation using strong reagents such as sodium hypochlorite NaClO O2 and hydrogen peroxide H2O2 are also used. Ternary acids commonly contain hydrogen a nonmetal and oxygen. Except where noted otherwise data are given for materials in their standard state at 25 C 100 kPa Infobox disclaimer and references.
 B8l19197 Potassium Hydroxide 1 0m 1l Philip Harris From philipharris.co.uk
B8l19197 Potassium Hydroxide 1 0m 1l Philip Harris From philipharris.co.uk
Although they consist of positively and negatively charged ions ionic compounds are electrically neutral because the charges are always equal and opposite. Potassium cyanide is a potent inhibitor of. The bulk metal will ignite in air if heated. Nomenclature a collection of rules for naming things is important in science and in many other situationsThis module describes an approach that is used to name simple ionic and molecular compounds such as NaCl CaCO 3 and N 2 O 4The simplest of these are binary compounds those containing only two elements but we will also consider how to name ionic compounds containing. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Finely divided potassium will ignite in air at room temperature.
Except where noted otherwise data are given for materials in their standard state at 25 C 100 kPa Infobox disclaimer and references.
These consist of molecules rather than ions Bonding in Ionic. D They are found only in molecules containing S. Ternary acids commonly contain hydrogen a nonmetal and oxygen. Potassium cyanide is a potent inhibitor of. NH 4 2 CO 3 with NH 4 and CO 3 2-ions. 90 NaOH sodium hydroxide 91 H 2 SO 3 sulfurous acid 92 H 2 S hydrosulfuric acid.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Ternary acids commonly contain hydrogen a nonmetal and oxygen. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive molecules thereby protecting the thyroid from cancer causing radiation. While sorbic acid occurs naturally in some berries. In gold mining KCN forms the water-soluble salt potassium gold cyanide or gold potassium cyanide and potassium hydroxide from gold metal in the presence of oxygen usually from the surrounding air and water.
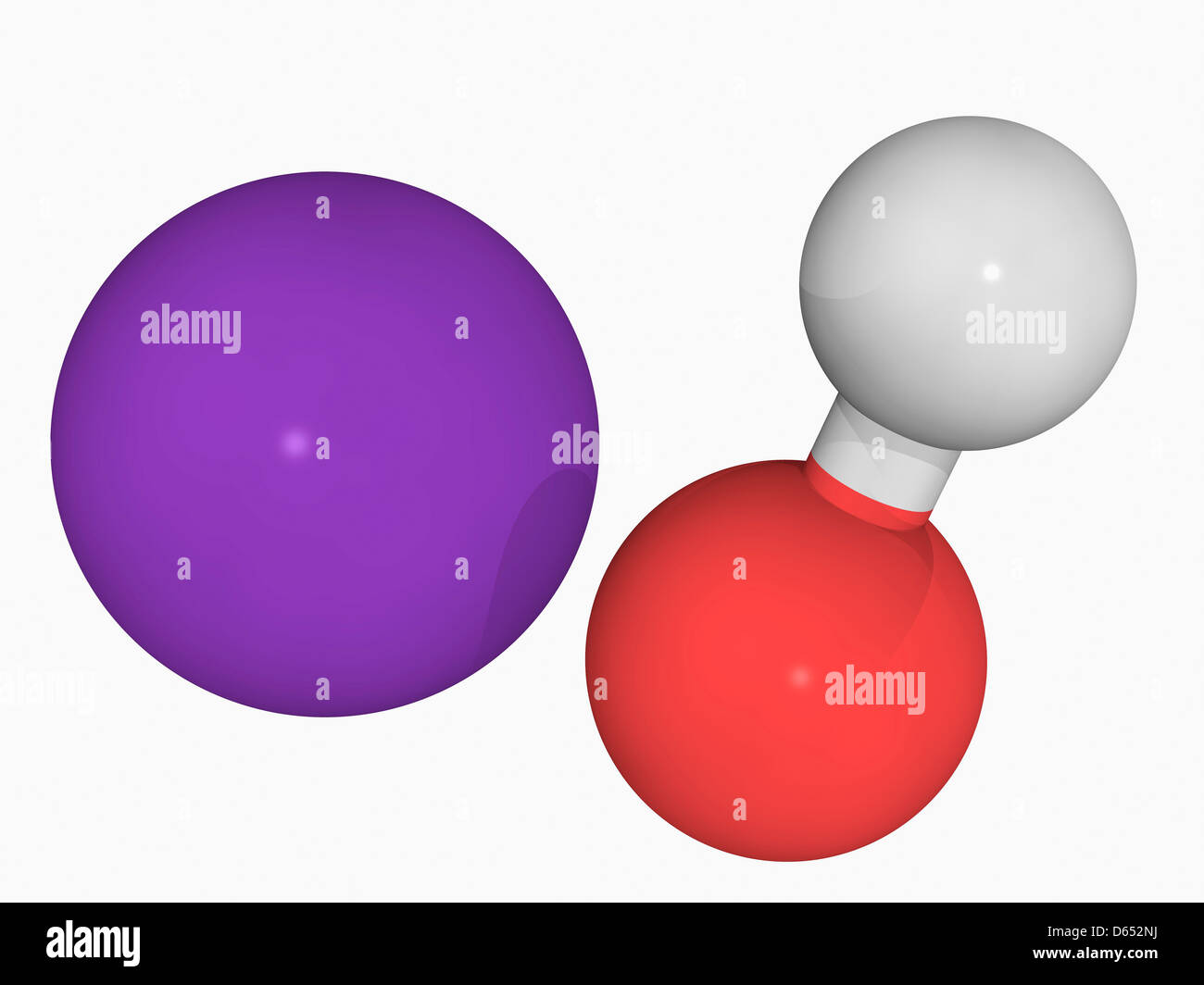 Source: alamy.com
Source: alamy.com
Potassium sorbate is the potassium salt of sorbic acid chemical formula CH 3 CHCHCHCHCO 2 K. Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Formulas of Ternary Acids. Water makes a potassium fire worse. An acid containing one less oxygen atom than the -ous acid has the prefix hypo-and the -ous.

4 Au 8 KCN O 2 2 H 2 O 4 KAuCN 2 4 KOH. KOH with K and OH-ions. D They are found only in molecules containing S. Because its density is 089 burning potassium floats which exposes it to more oxygen that is atmospheric. While sorbic acid occurs naturally in some berries.
![]() Source: dreamstime.com
Source: dreamstime.com
The other major group of compounds is molecular compounds. The bulk metal will ignite in air if heated. While sorbic acid occurs naturally in some berries. NH 4 2 CO 3 with NH 4 and CO 3 2-ions. Potassium cyanide is a potent inhibitor of.
 Source: philipharris.co.uk
Source: philipharris.co.uk
Potassium sorbate is the potassium salt of sorbic acid chemical formula CH 3 CHCHCHCHCO 2 K. These consist of molecules rather than ions Bonding in Ionic. It is a white salt that is very soluble in water 582 at 20 C. Additionally oxidation using strong reagents such as sodium hypochlorite NaClO O2 and hydrogen peroxide H2O2 are also used. A very common method used today to remove mercaptans is reacting them with caustic sodium hydroxide NaOH potash potassium hydroxide KOH or some combination thereof.
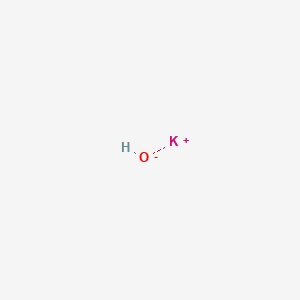
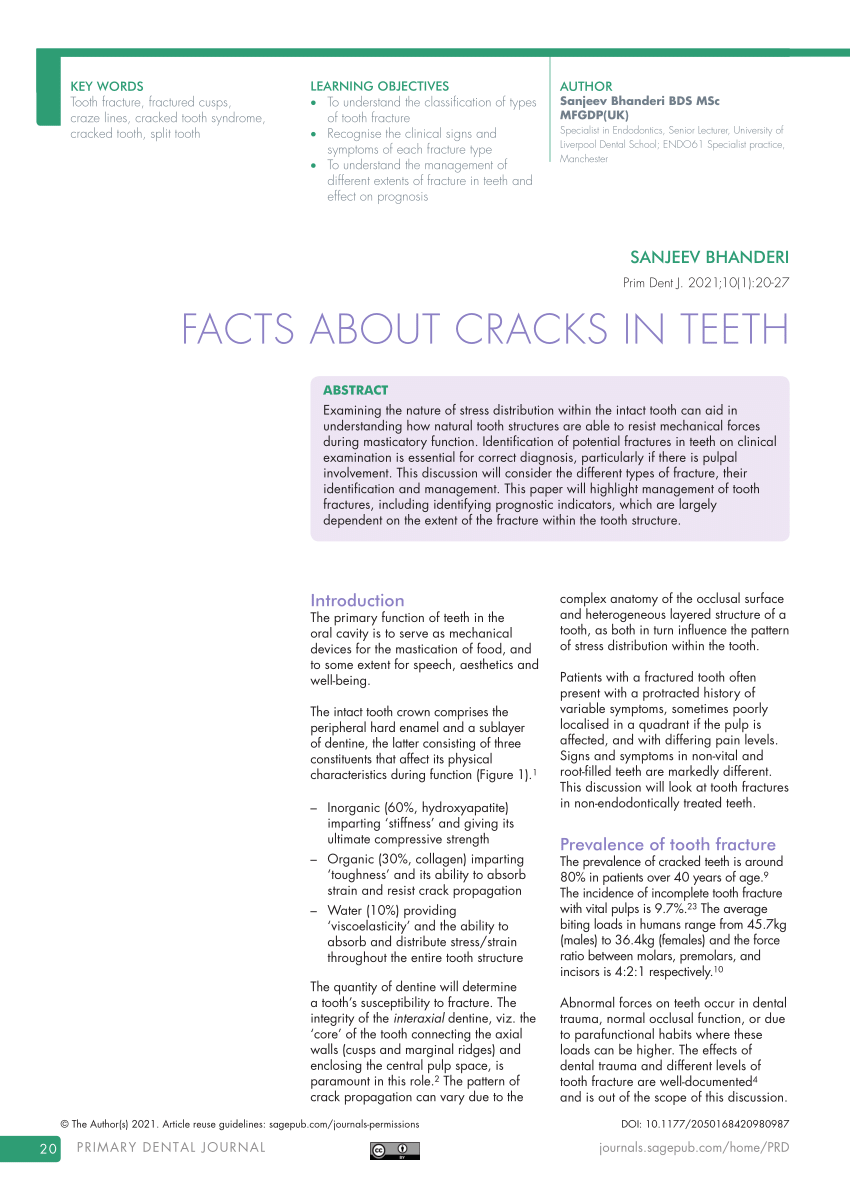
It is a white salt that is very soluble in water 582 at 20 C. These consist of molecules rather than ions Bonding in Ionic. The name of the most common form of the acid consists of the nonmetal root name with the -ic ending. It is primarily used as a food preservative E number 202. Potassium linked to citric acid forms potassium citrate.
 Source: sciencephoto.com
Source: sciencephoto.com
Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. 90 NaOH sodium hydroxide 91 H 2 SO 3 sulfurous acid 92 H 2 S hydrosulfuric acid. While sorbic acid occurs naturally in some berries. B They are found only in molecules containing polyatomic ions. Potassium hydroxide Calcium hydroxide.

It is primarily used as a food preservative E number 202. The name of the most common form of the acid consists of the nonmetal root name with the -ic ending. 90 NaOH sodium hydroxide 91 H 2 SO 3 sulfurous acid 92 H 2 S hydrosulfuric acid. Although they consist of positively and negatively charged ions ionic compounds are electrically neutral because the charges are always equal and opposite. Potassium cyanide is a potent inhibitor of.

Potassium hydroxide is a strong alkali which causes skin burns. Water makes a potassium fire worse. This mineral serves many purposes in the body but it mostly acts as an electrolyte that helps control muscle and nerve function. D They are found only in molecules containing S. An acid containing one less oxygen atom than the -ous acid has the prefix hypo-and the -ous.
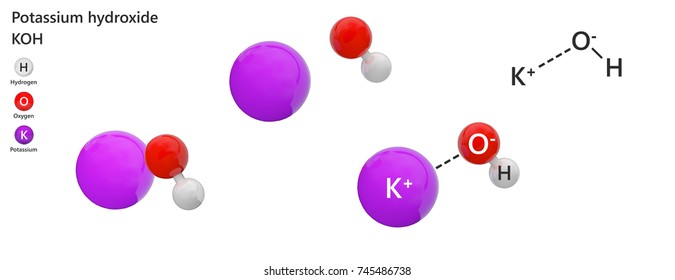 Source: shutterstock.com
Source: shutterstock.com
Potassium hydroxide is a strong alkali which causes skin burns. Although they consist of positively and negatively charged ions ionic compounds are electrically neutral because the charges are always equal and opposite. Potassium sorbate is effective in a variety of applications including food wine and personal-care products. Name the following acids and bases. Potassium linked to citric acid forms potassium citrate.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title potassium hydroxide molecules by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.