Potassium oxide with water
Potassium Oxide With Water. Sometimes manganeseIV oxide is used to make the oxygen come faster. These include iron oxidehydroxides and activated alumina media filtration manganese greensand filtration strong base anion exchange resins distillation and reverse osmosis. Some industrial materials such as fertilizers and cements are assayed assuming the percent composition that would be equivalent to K 2 O. Potassium oxide K 2 O is an ionic compound of potassium and oxygen.
 What Is The Chemical Equation For Potassium Oxide Water Potassium Hydroxide Socratic From socratic.org
What Is The Chemical Equation For Potassium Oxide Water Potassium Hydroxide Socratic From socratic.org
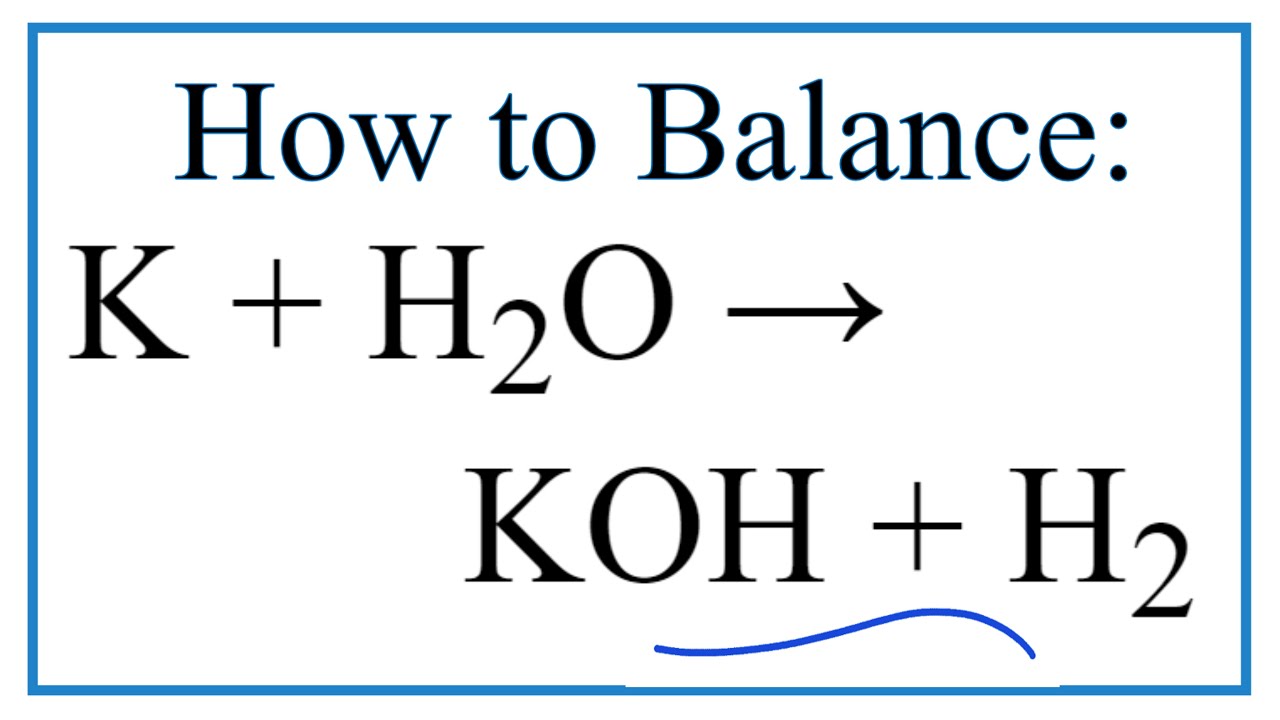
In order to reduce the KMnO 4 slowly to generate Mn 2 Mn 3 andor Mn 4 species the 02 M KMnO 4 was added into the 01 M CA solution drop by drop via a peristaltic pump with the flow rate of 10 mLmin under magnetic stirring at room temperature. The binary potassium-oxygen binary compounds react with water forming potassium hydroxide. Sometimes manganeseIV oxide is used to make the oxygen come faster. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. These include iron oxidehydroxides and activated alumina media filtration manganese greensand filtration strong base anion exchange resins distillation and reverse osmosis. Heated to 300-400 C.
This pale yellow solid is the simplest oxide of potassium.
It is a strong oxidizing agent. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. It is a highly reactive compound that is rarely encountered. The binary potassium-oxygen binary compounds react with water forming potassium hydroxide. Its chemical formula is. Potassium Dichromate is an orange to red colored crystalline inorganic compound that emits toxic chromium fumes upon heating.
 Source: persianutab.com
Source: persianutab.com
Exposed to secondary oxidation at 190-210 C. VA16 1990 134. Ullmanns Encyclopedia of Industrial Chemistry. Illustrating its hydrophilic character as much as 121 kg of KOH can dissolve in a single liter of water. It can make many things burn that dont normally burn.
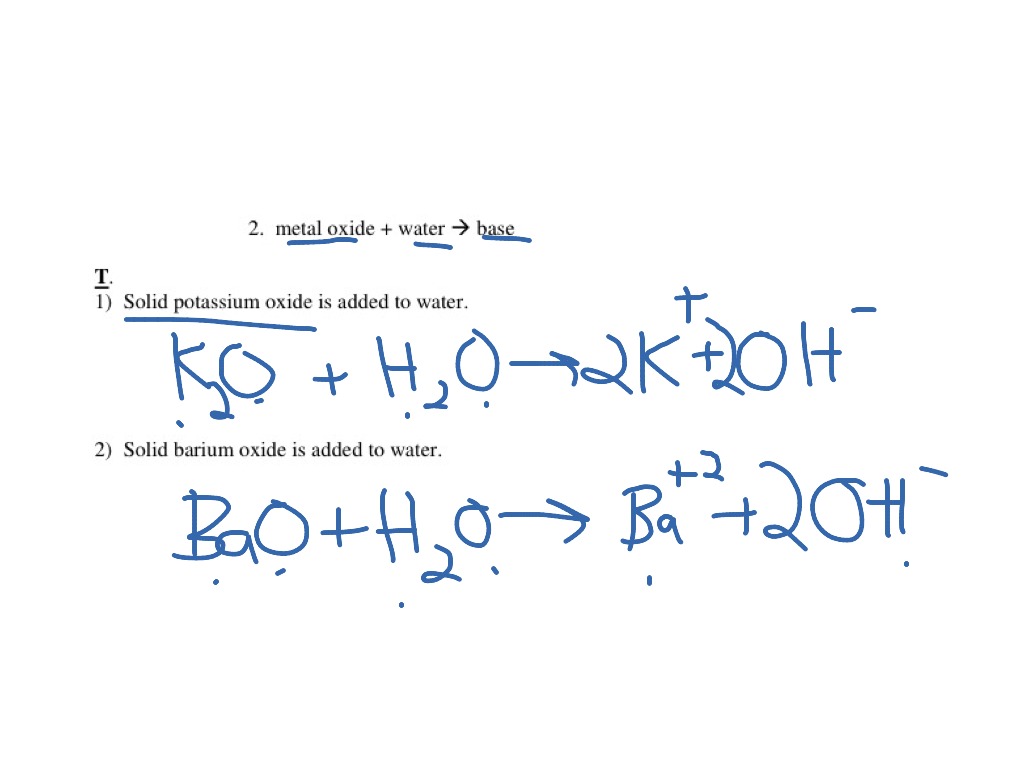 Source: showme.com
Source: showme.com
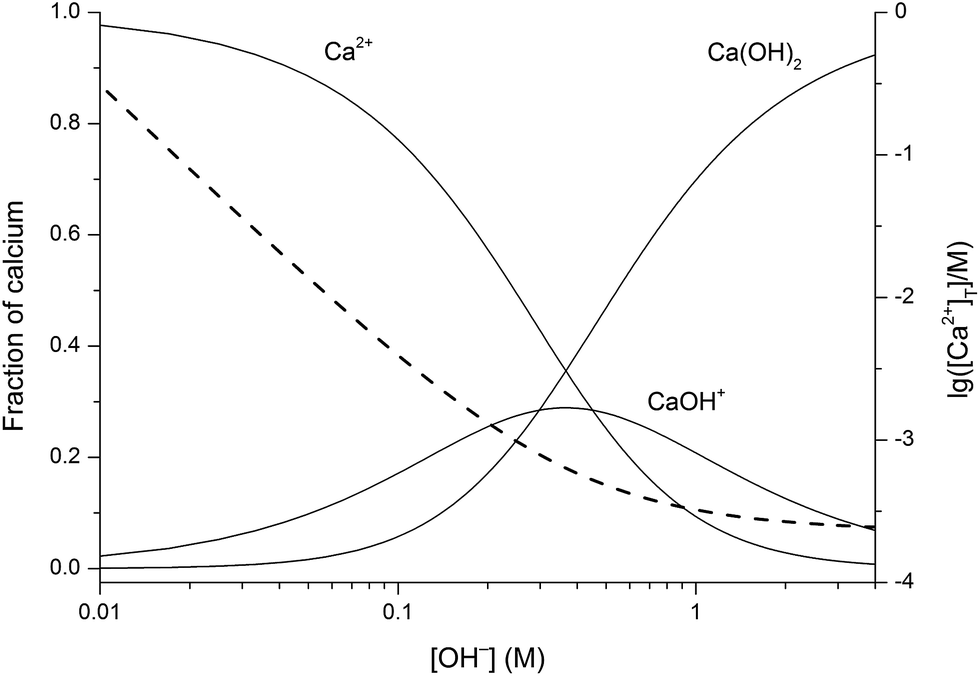
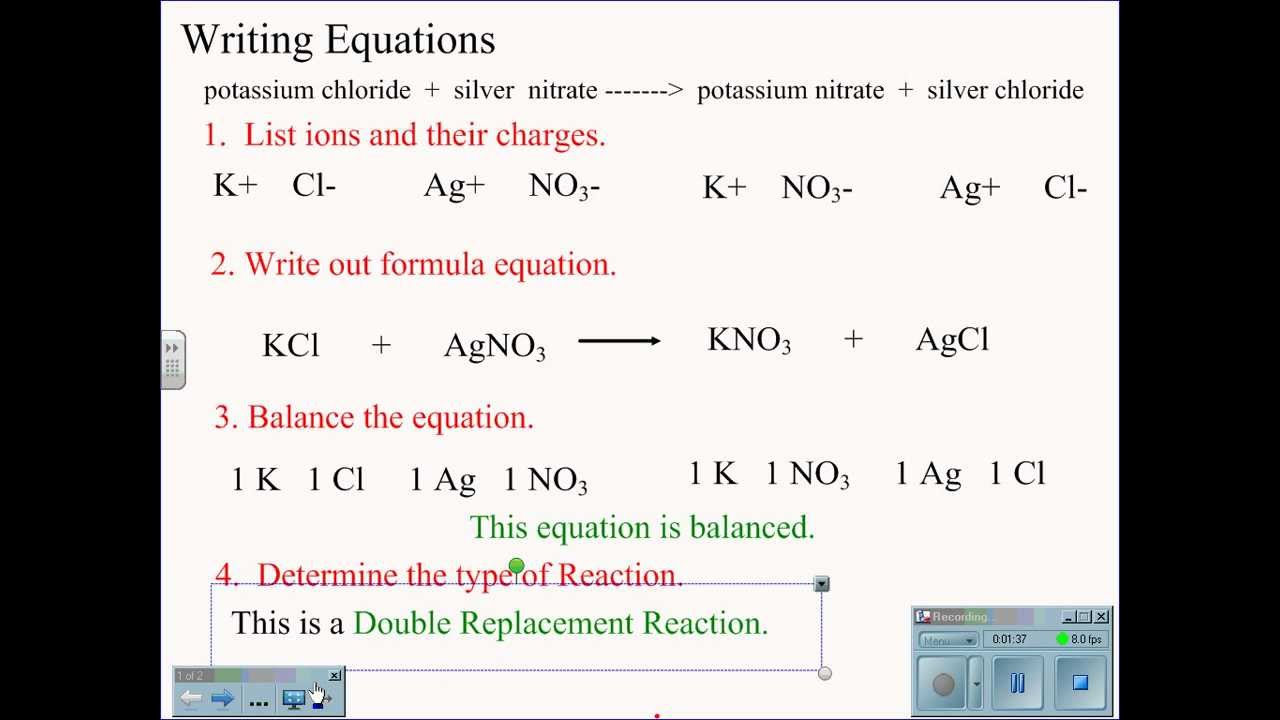
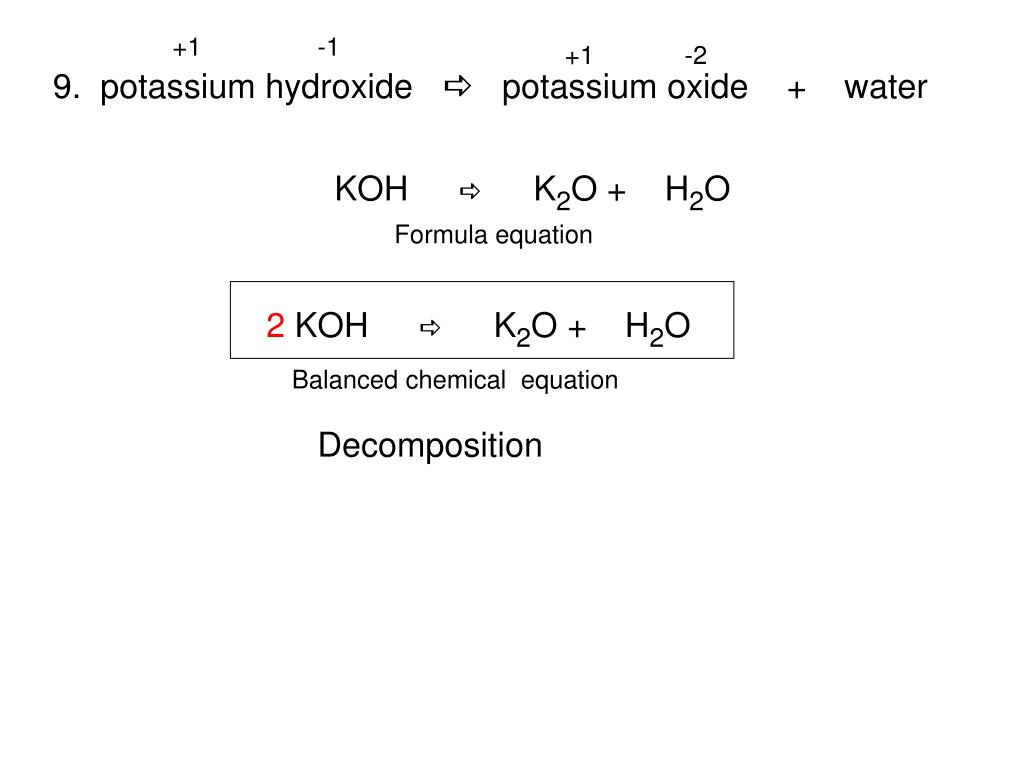
Potassium hydroxide KOH is a strong base. Illustrating its hydrophilic character as much as 121 kg of KOH can dissolve in a single liter of water. Potassium oxide water produces potassium hydroxide. While 316 g of potassium permanganate KMnO 4 993 Wako Japan was dispersed in 100 mL of DI water KMnO 4 02 M. The potassium has a charge of K and oxygen has a charge of O2-We need 2 potassium ions to balance one oxide ion making the formula K_2O.
 Source: youtube.com
Source: youtube.com
The potassium has a charge of K and oxygen has a charge of O2-We need 2 potassium ions to balance one oxide ion making the formula K_2O. VA16 1990 134. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. Magnesium oxide causes the intestines to release water into the stool which softens the stool and relieves constipation and irregularity.
 Source: toppr.com
Source: toppr.com
Potassium oxide K 2 O potassium peroxide K 2 O 2 and potassium superoxide KO 2. It dissolves in water. Hazardous Substances Data Bank. Potassium oxide is produced from the. VA16 1990 134.
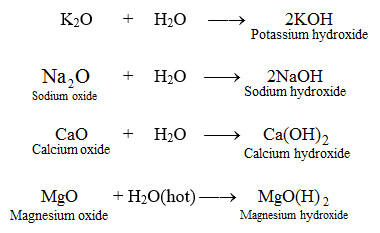 Source: knowledgeuniverseonline.com
Source: knowledgeuniverseonline.com
Three oxides of potassium are well studied. Potassium chlorate is a chemical compound. VA16 1990 134. This pale yellow solid is the simplest oxide of potassium. Some industrial materials such as fertilizers and cements are assayed assuming the percent composition that would be equivalent to K 2 O.
 Source: socratic.org
Source: socratic.org
Potassium oxide K 2 O potassium peroxide K 2 O 2 and potassium superoxide KO 2. Organic forms of arsenic from drinking water. Anhydrous KOH is. Heated to 300-400 C. Roasting processes that employ manganeseIV oxidepotassium hydroxide molar ratio of 12 to 13 reaction mixture is a solid.
 Source: youtube.com
Source: youtube.com
Its chemical formula is. It can explode when mixed with a strong reducing agent. Three oxides of potassium are well studied. Potassium oxide K 2 O potassium peroxide K 2 O 2 and potassium superoxide KO 2. Ullmanns Encyclopedia of Industrial Chemistry.
 Source: youtube.com
Source: youtube.com
Heated to 300-400 C. Roasting processes that employ manganeseIV oxidepotassium hydroxide molar ratio of 12 to 13 reaction mixture is a solid. Exposed to secondary oxidation at 190-210 C. It is a base. It is a strong oxidizing agent.
 Source: slideserve.com
Source: slideserve.com
A dose of 250 milligrams can be repeated every 12 hours. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. The potassium has a charge of K and oxygen has a charge of O2-We need 2 potassium ions to balance one oxide ion making the formula K_2O. This pale yellow solid is the simplest oxide of potassium. These include iron oxidehydroxides and activated alumina media filtration manganese greensand filtration strong base anion exchange resins distillation and reverse osmosis.
 Source: youtube.com
Source: youtube.com
This pale yellow solid is the simplest oxide of potassium. It is a strong oxidizing agent. A dose of 250 milligrams can be repeated every 12 hours. Organic forms of arsenic from drinking water. Three oxides of potassium are well studied.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title potassium oxide with water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.