Potassium water explosion
Potassium Water Explosion. The heat produced by this reaction may ignite the hydrogen or the metal itself resulting in a fire or an explosion. A highly oxidative water-soluble compound with purple crystals and a sweet taste. Do NOT induce vomiting. Immediately flush eyes with plenty of water for at least 15 minutes occasionally lifting the upper and lower eyelids.
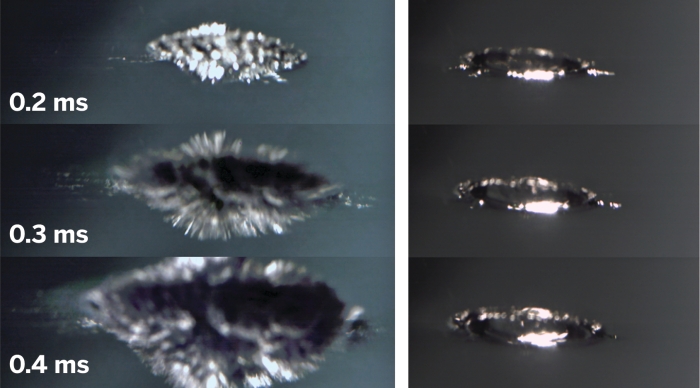 Why Sodium And Potassium Really Explode In Water From cen.acs.org
Why Sodium And Potassium Really Explode In Water From cen.acs.org
NaK is highly reactive with water like its constituent elements and may catch fire when exposed to air so must be handled. BEFORE WORKING WITH ALKALI METALS. A mixture of potassium chlorate and sodium amide explodes. If conscious and alert. There is no chemical reaction but there ia a reaction in that the disassociation of the sodium and hydroxide ions and the hydration of those ions releases a LOT of heat enough to boil water in some circumstances. Do NOT induce vomiting.
Immediate medical attention is required.
The heat produced by this reaction may ignite the hydrogen or the metal itself resulting in a fire or an explosion. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Various commercial grades are available. An explosion occurred during heating of a mixture of potassium chlorate and magnesium Chem. Ammonium nitrate can also be used in place of potassium nitrate but it tends to produce a concussive-type explosion that shatters bullets gun barrels rocks and anything else near the explosion. Sodiumpotassium alloy colloquially called NaK commonly pronounced n æ k is an alloy of the alkali metals sodium Na atomic number 11 and potassium K atomic number 19 that is normally liquid at room temperature.
 Source: youtube.com
Source: youtube.com
Great care is needed. Alkali metals react with water to produce heat hydrogen gas and the corresponding metal hydroxide. First-aid measures Eye Contact Rinse immediately with plenty of water also under the eyelids for at least 15 minutes. Develop a Standard Operating Procedure SOP for your. Sodiumpotassium alloy colloquially called NaK commonly pronounced n æ k is an alloy of the alkali metals sodium Na atomic number 11 and potassium K atomic number 19 that is normally liquid at room temperature.
 Source: arstechnica.com
Source: arstechnica.com
The metal reacts with the water first breaking the hydrogen bonds in the water and producing hydrogen gas. Gaseous ammonia mixed with air reacts so vigorously with potassium chlorate that the reaction could become dangerous Mellor 8217. NaK is highly reactive with water like its constituent elements and may catch fire when exposed to air so must be handled. BEFORE WORKING WITH ALKALI METALS. An explosion occurred during heating of a mixture of potassium chlorate and magnesium Chem.
 Source: ehs.stanford.edu
Source: ehs.stanford.edu
Contact with sulfuric acid may cause fire or explosion. Of course in some instances this may be the type of reaction you desire. When an alkali metal is dropped into water it produces an explosion of which there are two separate stages. Get medical aid immediately. Get medical aid immediately.
 Source: youtube.com
Source: youtube.com
The metal reacts with the water first breaking the hydrogen bonds in the water and producing hydrogen gas. If conscious and alert. Great care is needed. Skin Contact Wash off immediately with plenty of water for at least 15 minutes. Answer 1 of 14.
 Source: science.wonderhowto.com
Source: science.wonderhowto.com
1 Structures Expand this. Gaseous ammonia mixed with air reacts so vigorously with potassium chlorate that the reaction could become dangerous Mellor 8217. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Alkali metals react with water to produce heat hydrogen gas and the corresponding metal hydroxide. Used to make other chemicals and as a disinfectant.
 Source: youtube.com
Source: youtube.com
Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid immediately. NaK is highly reactive with water like its constituent elements and may catch fire when exposed to air so must be handled. Various commercial grades are available. This takes place faster for the more reactive heavier alkali metals.
 Source: scitechantiques.com
Source: scitechantiques.com
If conscious and alert. A highly oxidative water-soluble compound with purple crystals and a sweet taste. Various commercial grades are available. There is no chemical reaction but there ia a reaction in that the disassociation of the sodium and hydroxide ions and the hydration of those ions releases a LOT of heat enough to boil water in some circumstances. Get medical aid imme diately.
 Source: arstechnica.com
Source: arstechnica.com
Second the heat generated by the first part of the reaction often ignites the hydrogen gas causing it to. How can I protect myself. NaK is highly reactive with water like its constituent elements and may catch fire when exposed to air so must be handled. The metal reacts with the water first breaking the hydrogen bonds in the water and producing hydrogen gas. Alkali metals react with water to produce heat hydrogen gas and the corresponding metal hydroxide.
 Source: youtube.com
Source: youtube.com
The heavier alkali metals will react more violently with water. Answer 1 of 14. Permanganic acid HMnO4 potassium salt. BEFORE WORKING WITH ALKALI METALS. There is no chemical reaction but there ia a reaction in that the disassociation of the sodium and hydroxide ions and the hydration of those ions releases a LOT of heat enough to boil water in some circumstances.
 Source: cen.acs.org
Source: cen.acs.org
Get medical aid immediately. The heat produced by this reaction may ignite the hydrogen or the metal itself resulting in a fire or an explosion. A mixture of potassium chlorate and sodium amide explodes. Answer 1 of 14. There is no chemical reaction but there ia a reaction in that the disassociation of the sodium and hydroxide ions and the hydration of those ions releases a LOT of heat enough to boil water in some circumstances.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title potassium water explosion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.