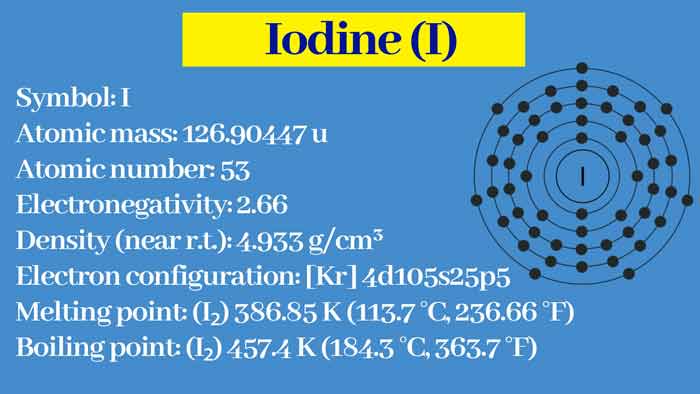
Properties of iodine
Properties Of Iodine. This element is predicted to be a liquid but is still a nonmetal. Possibly element 117 tennessine although most scientists think this element will behave as a metalloid. Iodine-125 125 I is a radioisotope of iodine which has uses in biological assays nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions including prostate cancer uveal melanomas and brain tumorsIt is the second longest-lived radioisotope of iodine after iodine-129. Moreover since iodine is a solid at room temperature it doesnt pose an explosion risk or need heavy high-pressure storage tanks promising simpler and miniaturised iodine-based systems.
 Iodine Properties Preparation And Uses From chemistrypage.in
Iodine Properties Preparation And Uses From chemistrypage.in
The material is crystalline and therefore the team was able to determine its structure using X-ray crystallography. Over the past few decades numerous reports on the use of povidone iodine have been published. Nonmetals have high ionization energies and electronegativities. Using iodine to test for the presence of starch is a common experiment. It is the heaviest stable member of its groupThe fifth and sixth halogens the radioactive astatine and tennessine are not well-studied due to their expense and inaccessibility in large quantities but appear to show various unusual properties for the group due to. Its half-life is 5949 days and it decays by electron capture to an excited.
Its half-life is 5949 days and it decays by electron capture to an excited.
Over the past few decades numerous reports on the use of povidone iodine have been published. Theres not much point in reading this page unless you are. Moreover since iodine is a solid at room temperature it doesnt pose an explosion risk or need heavy high-pressure storage tanks promising simpler and miniaturised iodine-based systems. This element is predicted to be a liquid but is still a nonmetal. The value of an extensive property is directly proportional to the amount of matter in question. An explanation of the physical properties of simple molecular substances including iodine ice and polythene.

Theres not much point in reading this page unless you are. They found nearly linear polyiodide chains in-between stacks of. The value of an extensive property is directly proportional to the amount of matter in question. This page describes how the physical properties of substances having molecular structures varies with the type of intermolecular attractions - hydrogen bonding or van der Waals forces. Using iodine to test for the presence of starch is a common experiment.
 Source: chemistrypage.in
Source: chemistrypage.in
This element is predicted to be a liquid but is still a nonmetal. The material is crystalline and therefore the team was able to determine its structure using X-ray crystallography. Theres not much point in reading this page unless you are. They found nearly linear polyiodide chains in-between stacks of. For instance a gallon of milk has a larger mass and volume than a cup of milk.
 Source: researchgate.net
Source: researchgate.net
The team investigated a related system a pyrroloperyleneiodine complex to study its properties as an organic electronic conductor. For instance a gallon of milk has a larger mass and volume than a cup of milk. Over the past few decades numerous reports on the use of povidone iodine have been published. Iodine-125 125 I is a radioisotope of iodine which has uses in biological assays nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions including prostate cancer uveal melanomas and brain tumorsIt is the second longest-lived radioisotope of iodine after iodine-129. Using iodine to test for the presence of starch is a common experiment.
 Source: researchgate.net
Source: researchgate.net
Nonmetals have high ionization energies and electronegativities. Using iodine to test for the presence of starch is a common experiment. The material is crystalline and therefore the team was able to determine its structure using X-ray crystallography. The team investigated a related system a pyrroloperyleneiodine complex to study its properties as an organic electronic conductor. Possibly element 117 tennessine although most scientists think this element will behave as a metalloid.

It is the heaviest stable member of its groupThe fifth and sixth halogens the radioactive astatine and tennessine are not well-studied due to their expense and inaccessibility in large quantities but appear to show various unusual properties for the group due to. It is the heaviest stable member of its groupThe fifth and sixth halogens the radioactive astatine and tennessine are not well-studied due to their expense and inaccessibility in large quantities but appear to show various unusual properties for the group due to. Nonmetals have high ionization energies and electronegativities. This page describes how the physical properties of substances having molecular structures varies with the type of intermolecular attractions - hydrogen bonding or van der Waals forces. Povidone iodines broad spectrum of activity ability to penetrate biofilms lack of associated resistance anti-inflammatory properties low cytotoxicity and good tolerability have been cited as important factors and no negative effect on wound healing has been observed in clinical practice.
 Source: haikudeck.com
Source: haikudeck.com
They found nearly linear polyiodide chains in-between stacks of. Iodine is the fourth halogen being a member of group 17 in the periodic table below fluorine chlorine and bromine. This element is predicted to be a liquid but is still a nonmetal. Iodine is much more abundant and cheaper than xenon and has three times the density of xenon even when the gas is pressurised. An explanation of the physical properties of simple molecular substances including iodine ice and polythene.
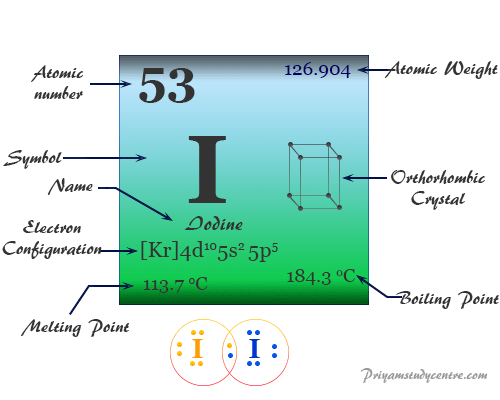 Source: priyamstudycentre.com
Source: priyamstudycentre.com
Nonmetals have high ionization energies and electronegativities. This element is predicted to be a liquid but is still a nonmetal. Povidone iodines broad spectrum of activity ability to penetrate biofilms lack of associated resistance anti-inflammatory properties low cytotoxicity and good tolerability have been cited as important factors and no negative effect on wound healing has been observed in clinical practice. Properties of matter fall into one of two categories. This page describes how the physical properties of substances having molecular structures varies with the type of intermolecular attractions - hydrogen bonding or van der Waals forces.
 Source: britannica.com
Source: britannica.com
Moreover since iodine is a solid at room temperature it doesnt pose an explosion risk or need heavy high-pressure storage tanks promising simpler and miniaturised iodine-based systems. Iodine-125 125 I is a radioisotope of iodine which has uses in biological assays nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions including prostate cancer uveal melanomas and brain tumorsIt is the second longest-lived radioisotope of iodine after iodine-129. If the property depends on the amount of matter present it is an extensive property. Iodine is much more abundant and cheaper than xenon and has three times the density of xenon even when the gas is pressurised. Its half-life is 5949 days and it decays by electron capture to an excited.
 Source: britannica.com
Source: britannica.com
Properties of Nonmetals. Iodine-125 125 I is a radioisotope of iodine which has uses in biological assays nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions including prostate cancer uveal melanomas and brain tumorsIt is the second longest-lived radioisotope of iodine after iodine-129. Properties of matter fall into one of two categories. The team investigated a related system a pyrroloperyleneiodine complex to study its properties as an organic electronic conductor. Iodine monochloride ICl or ClI CID 24640 - structure chemical names physical and chemical properties classification patents literature biological activities.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The mass and volume of a substance are examples of extensive properties. The noble gas elements are. This page describes how the physical properties of substances having molecular structures varies with the type of intermolecular attractions - hydrogen bonding or van der Waals forces. Moreover since iodine is a solid at room temperature it doesnt pose an explosion risk or need heavy high-pressure storage tanks promising simpler and miniaturised iodine-based systems. Povidone iodines broad spectrum of activity ability to penetrate biofilms lack of associated resistance anti-inflammatory properties low cytotoxicity and good tolerability have been cited as important factors and no negative effect on wound healing has been observed in clinical practice.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title properties of iodine by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.