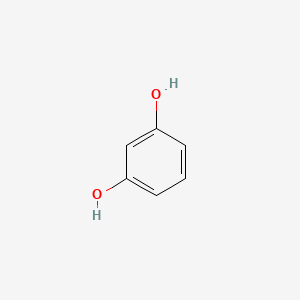
Resorcinol iupac name
Resorcinol Iupac Name. Finally if there are three or more substituent groups the ring is numbered in such a way as to assign the substituents the lowest possible numbers as illustrated by the last row of examples. The name is 68. The carbon atoms are numbered from the end closest to the OH group. It was first discovered by destructive distillation of the plant extract catechin.
 Resorcinol Wikipedia From en.wikipedia.org
Resorcinol Wikipedia From en.wikipedia.org
HOCH 2 CH 2 CH 2 CH 2 CH 2 OH. Depolarization of the neuronal membrane is inhibited thereby blocking the initiation and conduction of nerve impulses. Which of the following is dihydric alcohol. A Benzyl alcohol B Cyclohexanol C Phenol D m-Chlorophenol. Give the IUPAC name for each compound. A acetic acid b methyl alcohol c diethyl ether d acetone.
Catechol ˈ k æ t ɪ tʃ ɒ l or ˈ k æ t ɪ k ɒ l also known as pyrocatechol or 12-dihydroxybenzene is a toxic organic compound with the molecular formula C 6 H 4 OH 2It is the ortho isomer of the three isomeric benzenediolsThis colorless compound occurs naturally in trace amounts.
Common and IUPAC Names of Some Ethers. This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-1-Naphthylethylenediamine resulting in an azo dye and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution. Common and IUPAC Names of Some Ethers. Which of the following is most acidic. It was first discovered by destructive distillation of the plant extract catechin. The carbon atoms are numbered from the end closest to the OH group.
 Source: researchgate.net
Source: researchgate.net
Common names of ethers are derived from the names of alkyl aryl groups written as separate words in alphabetical order and adding the word ether at the end. The substituents are. An example of a compound with functional group O is. A Benzyl alcohol B Cyclohexanol C Phenol D m-Chlorophenol. In general an IUPAC name will have three essential features.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Common name Catechol Benzene-12-diol Resorcinol Benzene-13-diol Hydroquinone or quinol IUPAC name Benzene-14-diol c Ethers. A Benzyl alcohol B Cyclohexanol C Phenol D m-Chlorophenol. Common and IUPAC Names of Some Ethers. IUPAC name of m-cresol is _____ a 2-methylphenol b 3-chlorophenol c 3-methoxyphenol d benzene-1 3-diol. As the compound readily forms diazo compounds it is used to make dyes and sulfa drugs.
 Source: toppr.com
Source: toppr.com
As the compound readily forms diazo compounds it is used to make dyes and sulfa drugs. HOCH 2 CH 2 CH 2 CH 2 CH 2 OH. The carbon atoms are numbered from the end closest to the OH group. Ten carbon atoms in the LCC makes the compound a derivative of decane rule 1 and the OH on the third carbon atom makes it a 3-decanol rule 2. Check Answer and Solution for above question.

An example of a compound with functional group O is. Catechol ˈ k æ t ɪ tʃ ɒ l or ˈ k æ t ɪ k ɒ l also known as pyrocatechol or 12-dihydroxybenzene is a toxic organic compound with the molecular formula C 6 H 4 OH 2It is the ortho isomer of the three isomeric benzenediolsThis colorless compound occurs naturally in trace amounts. Depolarization of the neuronal membrane is inhibited thereby blocking the initiation and conduction of nerve impulses. Common and IUPAC Names of Some Ethers. This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-1-Naphthylethylenediamine resulting in an azo dye and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Common and IUPAC Names of Some Ethers. It was first discovered by destructive distillation of the plant extract catechin. Finally if there are three or more substituent groups the ring is numbered in such a way as to assign the substituents the lowest possible numbers as illustrated by the last row of examples. The carbon atoms are numbered from the end closest to the OH group. In general an IUPAC name will have three essential features.
Levomenthol is a levo isomer of menthol an organic compound made synthetically or obtained from peppermint or mint oils with flavoring and local anesthetic propertiesWhen added to pharmaceuticals and foods menthol functions as a fortifier for peppermint flavors. A Benzyl alcohol B Cyclohexanol C Phenol D m-Chlorophenol. It also has a counterirritant effect on skin and mucous membranes thereby producing a local analgesic or anesthetic effect. In general an IUPAC name will have three essential features. An example of a compound with functional group O is.
 Source: molinstincts.com
Source: molinstincts.com
Common names of ethers are derived from the names of alkyl aryl groups written as separate words in alphabetical order and adding the word ether at the end. Which of the following is most acidic. Finally if there are three or more substituent groups the ring is numbered in such a way as to assign the substituents the lowest possible numbers as illustrated by the last row of examples. This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-1-Naphthylethylenediamine resulting in an azo dye and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution. For example CH3OC2H5 is ethylmethyl ether.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Levomenthol is a levo isomer of menthol an organic compound made synthetically or obtained from peppermint or mint oils with flavoring and local anesthetic propertiesWhen added to pharmaceuticals and foods menthol functions as a fortifier for peppermint flavors. A acetic acid b methyl alcohol c diethyl ether d acetone. In general an IUPAC name will have three essential features. Common and IUPAC Names of Some Ethers. Benzocaine is an ester of paraaminobenzoic acid lacking the terminal diethylamino group of procaine with anesthetic activityBenzocaine binds to the sodium channel and reversibly stabilizes the neuronal membrane which decreases its permeability to sodium ions.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Depolarization of the neuronal membrane is inhibited thereby blocking the initiation and conduction of nerve impulses. Ten carbon atoms in the LCC makes the compound a derivative of decane rule 1 and the OH on the third carbon atom makes it a 3-decanol rule 2. For example CH3OC2H5 is ethylmethyl ether. Which of the following is dihydric alcohol. Benzocaine is an ester of paraaminobenzoic acid lacking the terminal diethylamino group of procaine with anesthetic activityBenzocaine binds to the sodium channel and reversibly stabilizes the neuronal membrane which decreases its permeability to sodium ions.
 Source: degruyter.com
Source: degruyter.com
Common name Catechol Benzene-12-diol Resorcinol Benzene-13-diol Hydroquinone or quinol IUPAC name Benzene-14-diol c Ethers. Salicylic acid resorcinol. Levomenthol is a levo isomer of menthol an organic compound made synthetically or obtained from peppermint or mint oils with flavoring and local anesthetic propertiesWhen added to pharmaceuticals and foods menthol functions as a fortifier for peppermint flavors. As the compound readily forms diazo compounds it is used to make dyes and sulfa drugs. Which of the following is most acidic.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title resorcinol iupac name by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.