Sodium acetate strong or weak base
Sodium Acetate Strong Or Weak Base. This buffer will have an acidic pH. Tb reflect immunological memory and are enriched at the site of infection. The buffering ability and properties under dilution of acetic acid- sodium acetate buffers will be determined. Add 100 ml of 01M acetic acid solution to a medium beaker.

The base doesnt actually dissociate K b. They may be similar in odor to the acid or base they are derived from. Add 100 ml of 01M acetic acid solution to a medium beaker. Weak salts or weak electrolyte salts are as the name suggests composed of weak electrolytes. Although both substances are acids you wouldnt use muriatic acid in salad dressing and vinegar is ineffective in cleaning bricks or concrete. Aviation History offers air enthusiasts the most detailed coverage of the history of manned flight with action-packed stories and illustrations that put the reader in the cockpit with pilots and military Army Navy and Marines aviators to experience aviations greatest dramas.
Aviation History offers air enthusiasts the most detailed coverage of the history of manned flight with action-packed stories and illustrations that put the reader in the cockpit with pilots and military Army Navy and Marines aviators to experience aviations greatest dramas.
Calculate the mass of solid sodium acetate that must be added to the acetic acid solution to bring the pH to 475. NH 3 RNH 2 etc. The Common-Ion Effect example of Le Chateliers Principle the shift in an equilibrium caused by the addition or removal of one of the species participating in the equilibrium. Add 100 ml of 01M acetic acid solution to a medium beaker. For example sodium acetate NaCH. Record the mass and calculations in the data sheet.
 Source: slideplayer.com
Source: slideplayer.com
The base doesnt actually dissociate K b. Aviation History offers air enthusiasts the most detailed coverage of the history of manned flight with action-packed stories and illustrations that put the reader in the cockpit with pilots and military Army Navy and Marines aviators to experience aviations greatest dramas. MD Professor and Vice Chair for Research Division Head Infectious Disease Wayne L. Introduction A buffer is a solution that resists changes in pH upon. Weak Base Equilibria We will consider weak bases that are molecules containing a N with a lone pair.
 Source: youtube.com
Source: youtube.com
Weak salts or weak electrolyte salts are as the name suggests composed of weak electrolytes. A pH 5 or pH 9 buffer will be prepared using solid sodium acetate or ammonium chloride. The base doesnt actually dissociate K b. Answer 1 of 3. For example sodium acetate NaCH.
 Source: reddit.com
Source: reddit.com
Many hardware stores sell muriatic acid a 6 M solution of hydrochloric acid HClaq to clean bricks and concrete. NH 3 RNH 2 etc. Add the solid sodium acetate to the acetic acid solution. The buffering ability and properties under dilution of acetic acid- sodium acetate buffers will be determined. Tb reflect immunological memory and are enriched at the site of infection.

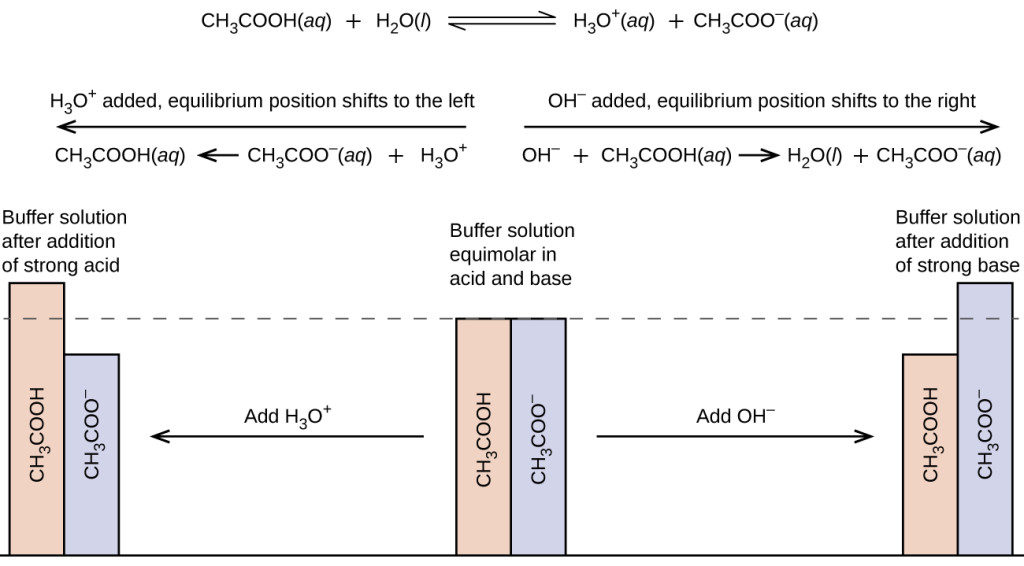
A mixture of a weak acid and its conjugate base or a mixture of a weak base and its conjugate acid is called a buffer solution or a bufferBuffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak acid and its salt. NH 3 RNH 2 etc. They are generally more volatile than strong salts. Strong and Weak Acids and Bases. The base doesnt actually dissociate K b.

Weak Base Equilibria We will consider weak bases that are molecules containing a N with a lone pair. Strong and Weak Acids and Bases. A buffer is a concentrated solution of a weak acid or base together with a salt containing the conjugate base or acid. Tb reflect immunological memory and are enriched at the site of infection. Aviation History magazine is an authoritative in-depth history of world aviation from its origins to the Space Age.
 Source: youtube.com
Source: youtube.com
Record the mass and calculations in the data sheet. Other weak bases also known however General equilibrium for a weak base in water This is a base-dissociation reaction or base-ionization reaction NOTE. They may be similar in odor to the acid or base they are derived from. How does a Buffer work. Aviation History offers air enthusiasts the most detailed coverage of the history of manned flight with action-packed stories and illustrations that put the reader in the cockpit with pilots and military Army Navy and Marines aviators to experience aviations greatest dramas.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
This buffer will have an acidic pH. CH 3 CO 2 H. Addition of sodium acetate CH 3COONa or NaAc to. NH 3 RNH 2 etc. The base doesnt actually dissociate K b.

The Common-Ion Effect example of Le Chateliers Principle the shift in an equilibrium caused by the addition or removal of one of the species participating in the equilibrium. Addition of sodium acetate CH 3COONa or NaAc to. The base doesnt actually dissociate K b. Other weak bases also known however General equilibrium for a weak base in water This is a base-dissociation reaction or base-ionization reaction NOTE. The buffering ability and properties under dilution of acetic acid- sodium acetate buffers will be determined.
 Source: slideplayer.com
Source: slideplayer.com
Add 100 ml of 01M acetic acid solution to a medium beaker. This buffer will have an acidic pH. Strong and Weak Acids and Bases. Other weak bases also known however General equilibrium for a weak base in water This is a base-dissociation reaction or base-ionization reaction NOTE. Weak Base Equilibria We will consider weak bases that are molecules containing a N with a lone pair.
 Source: xaktly.com
Source: xaktly.com
Calculate the mass of solid sodium acetate that must be added to the acetic acid solution to bring the pH to 475. Aviation History offers air enthusiasts the most detailed coverage of the history of manned flight with action-packed stories and illustrations that put the reader in the cockpit with pilots and military Army Navy and Marines aviators to experience aviations greatest dramas. Add 100 ml of 01M acetic acid solution to a medium beaker. Aviation History magazine is an authoritative in-depth history of world aviation from its origins to the Space Age. Acetil salicylic acid is an organic acid having one carboxilic group which confers the acidity to the acetil salicylic acid and then we can write it as C8H7COOH then a neutralization reaction of the acid with soda NaOH will be C8H7COOH NaOH C8H7COONa H2O or C9H8O4 Na.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium acetate strong or weak base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.