Sodium azide decomposition
Sodium Azide Decomposition. Sodium appears as a silvery soft metal that becomes grayish white upon exposure to air. NaN 3 300C 2Na 3N 2 NH 4 2 Cr 2 O 7 N 2 Cr 2 O 3 4H 2 O. Both reactions must only be carried out under controlled conditions by a professional. Sodium azide is made industrially by the reaction of nitrous oxide N 2 O with sodium amide in liquid ammonia as solvent.
 Question 670ca Socratic From socratic.org
Question 670ca Socratic From socratic.org
Both reactions must only be carried out under controlled conditions by a professional. It is an ionic substance is highly soluble in water and is very acutely poisonous. Burns violently with explosions that may spatter the material. Nitrogen is made on massive scale by liquefaction of air and fractional distillation of the resulting liquid air to. How many liters of nitrogen measured at 25 C and 100 atm will be produced by 1000 g of NaN3Equation. Recoveries of sodium azide from.
2NaN3s – 2Nas 3N2s.
The balanced equation for the decomposition of sodium azide is _____ 12311. The formula of nitrobenzene is C6H5NO2. Nitrogen is made on massive scale by liquefaction of air and fractional distillation of the resulting liquid air to. Both reactions must only be carried out under controlled conditions by a professional. Burns violently with explosions that may spatter the material. Sodium azide in a sample was acidified and the azide was converted to the volatile hydrazoic acid which was trapped in 25 mM sodium hydroxide solution.
 Source: youtube.com
Source: youtube.com
N 2 O 2 NaNH 2 NaN 3 NaOH NH 3. It is used for the preparation of other azide compounds. Calibration curves were linear for 05 to 20 ugmL sodium azide and the detection limit was 005 ugmL. Sodium azide is the inorganic compound with the formula NaN 3This colorless salt is the gas-forming component in legacy citation needed car airbag systems. Dissolve 2 grams of soluble starch and 02 grams of salicylic acid in 100 ml of hot.
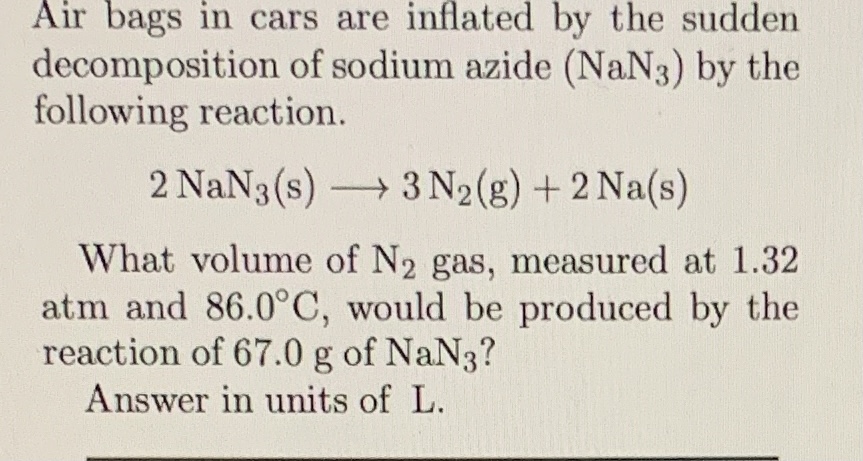 Source: bartleby.com
Source: bartleby.com
Sodium azide is the inorganic compound with the formula NaN 3This colorless salt is the gas-forming component in legacy citation needed car airbag systems. The molecular weight of this compound is _____ amu. Many inorganic azides can be prepared directly or indirectly from sodium azide. N 2 O 2 NaNH 2 NaN 3 NaOH NH 3. Shipped as a solid or molten liquid.
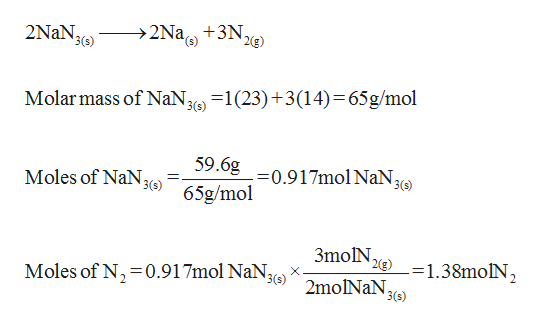 Source: bartleby.com
Source: bartleby.com
Sodium azide decomposes into solid sodium metal and nitrogen gas. 2NaN3s – 2Nas 3N2s. The molecular weight of this compound is _____ amu. Dissolve 2 grams of soluble starch and 02 grams of salicylic acid in 100 ml of hot. Dissolve 10 g sodium azide NaN3 in 40 ml water and add to alkali- iodide solution The resultant reagent should not contain free iodine check through diluting and acidifying and adding starch indicator and observe for blue color Concentrated sulfuric acid Aqueous solution of starch indicator.
 Source: socratic.org
Source: socratic.org
How many liters of nitrogen measured at 25 C and 100 atm will be produced by 1000 g of NaN3Equation. The molecular weight of this compound is _____ amu. How many liters of nitrogen measured at 25 C and 100 atm will be produced by 1000 g of NaN3Equation. Many inorganic azides can be prepared directly or indirectly from sodium azide. Calibration curves were linear for 05 to 20 ugmL sodium azide and the detection limit was 005 ugmL.

Nitrogen is made on massive scale by liquefaction of air and fractional distillation of the resulting liquid air to. Calibration curves were linear for 05 to 20 ugmL sodium azide and the detection limit was 005 ugmL. Determination was performed by isocratic ion chromatography using suppressed conductivity detection. Substance that dissociates into ions when dissolved in water. Both reactions must only be carried out under controlled conditions by a professional.
 Source: toppr.com
Source: toppr.com
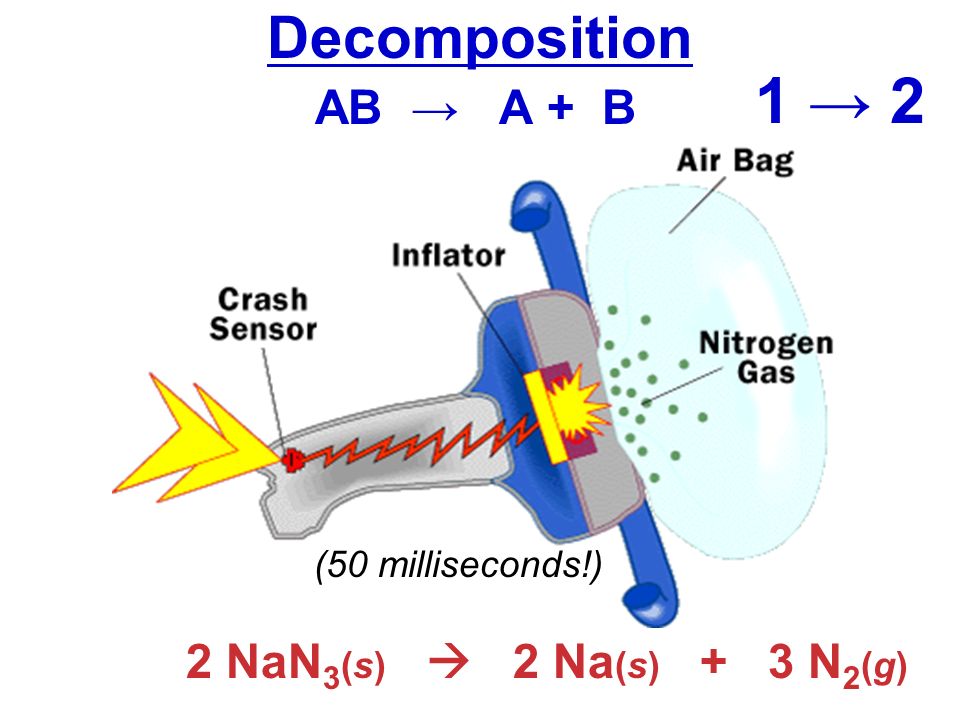
Nitrogen is made on massive scale by liquefaction of air and fractional distillation of the resulting liquid air to. The air bags that are currently installed in automobiles to prevent injuries in the event of a crash are equipped with sodium azide NaN3 which decomposes when activated by an electronic igniter to produce nitrogen gas that fills the bag. May dissolve in water but it. Dissolve 10 g sodium azide NaN3 in 40 ml water and add to alkali- iodide solution The resultant reagent should not contain free iodine check through diluting and acidifying and adding starch indicator and observe for blue color Concentrated sulfuric acid Aqueous solution of starch indicator. The formula weight of ammonium sulfate NH42SO4 rounded to the nearest integer is _____ amu.
 Source: youtube.com
Source: youtube.com
The formula weight of ammonium sulfate NH42SO4 rounded to the nearest integer is _____ amu. Sodium azide is made industrially by the reaction of nitrous oxide N 2 O with sodium amide in liquid ammonia as solvent. Both reactions must only be carried out under controlled conditions by a professional. Used for making gasoline additives electric power cable sodium lamps other chemicals. Determination was performed by isocratic ion chromatography using suppressed conductivity detection.
 Source: youtube.com
Source: youtube.com
Burns violently with explosions that may spatter the material. The formula weight of ammonium sulfate NH42SO4 rounded to the nearest integer is _____ amu. The molecular weight of this compound is _____ amu. The air bags that are currently installed in automobiles to prevent injuries in the event of a crash are equipped with sodium azide NaN3 which decomposes when activated by an electronic igniter to produce nitrogen gas that fills the bag. Burns violently with explosions that may spatter the material.
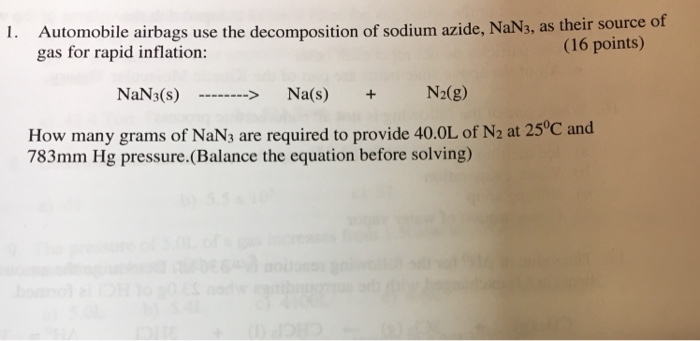
NaN 3 300C 2Na 3N 2 NH 4 2 Cr 2 O 7 N 2 Cr 2 O 3 4H 2 O. NaN 3 300C 2Na 3N 2 NH 4 2 Cr 2 O 7 N 2 Cr 2 O 3 4H 2 O. The formula weight of ammonium sulfate NH42SO4 rounded to the nearest integer is _____ amu. Used for making gasoline additives electric power cable sodium lamps other chemicals. 2NaN3s – 2Nas 3N2s.
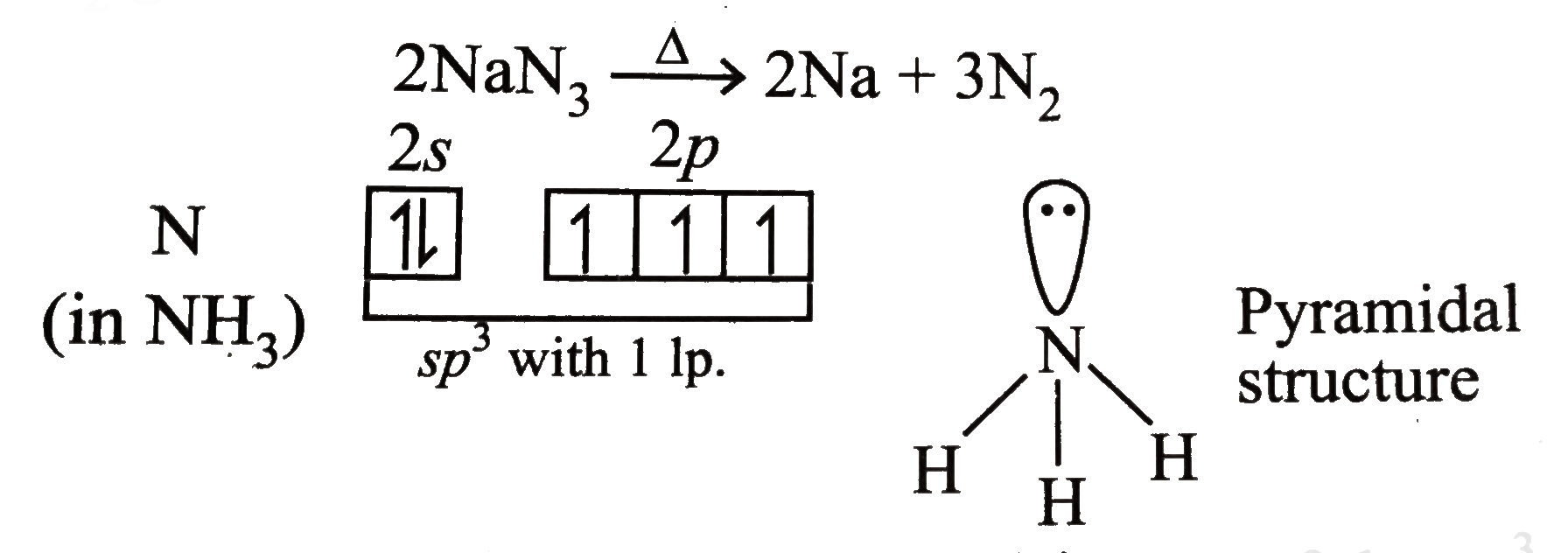 Source: doubtnut.app
Source: doubtnut.app
The molecular weight of this compound is _____ amu. Determination was performed by isocratic ion chromatography using suppressed conductivity detection. Dissolve 2 grams of soluble starch and 02 grams of salicylic acid in 100 ml of hot. NaN 3 300C 2Na 3N 2 NH 4 2 Cr 2 O 7 N 2 Cr 2 O 3 4H 2 O. How many liters of nitrogen measured at 25 C and 100 atm will be produced by 1000 g of NaN3Equation.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium azide decomposition by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.