Sodium bicarbonate acid or base
Sodium Bicarbonate Acid Or Base. Sodium carbonate has several uses in cuisine largely because it is a stronger base than baking soda sodium bicarbonate but weaker than lye which may refer to sodium hydroxide or less commonly potassium hydroxide. Bicarbonate ions and carbonic acid are present in the blood in a 201 ratio if the blood pH is within the normal range. Sodium bicarbonate has the chemical formula NaHCO3. In sum sodium bicarbonate should be reserved for severe cases of acidosis only pH.
 Is Baking Soda Nahco3 An Acid Or Base Or Both Nature Of Its Solution From topblogtenz.com
Is Baking Soda Nahco3 An Acid Or Base Or Both Nature Of Its Solution From topblogtenz.com
However when the acidosis results from organic acids lactic acid acetoacetic acid etc the role of bicarbonate is controversial. You found a. We would like to show you a description here but the site wont allow us. Sodium bicarbonate strong acid weak acid salt H 2 CO 3 NaOHHCO 3- H 2 O weak acid strong basebicarbonate water As with the phosphate buffer a weak acid or weak base captures the free ions and a significant change in pH is prevented. The gas released during an acid-base reaction between baking soda and an acid such as cream of tartar lemon juice or lactic acid in buttermilk causes baked goods to rise. In toothpaste sodium bicarbonate helps to remove stains from teeth by dislodging tiny particles of food or beverages that can blemish tooth enamel.
To take advantage of the.
To take advantage of the. Alkalinity affects gluten production in kneaded doughs and also improves browning by reducing the temperature at which the Maillard reaction occurs. This is very important in your bloodstream because to maintain cellular. Sodium bicarbonate strong acid weak acid salt H 2 CO 3 NaOHHCO 3- H 2 O weak acid strong basebicarbonate water As with the phosphate buffer a weak acid or weak base captures the free ions and a significant change in pH is prevented. The chemical reaction observed showed that there was fizzing and bubbling this is evidence that a new gas was being produced. After the fizzing stopped a liquid was leftover leading me to conclude the liquid leftover leading me to conclude the liquid leftover was the NaCl and H2O 4.

Its a salt with both acidic and basic properties but most importantly it acts as a chemical buffer. A buffer is a chemical that can react with small amounts of either acid or base helping to prevent changes in solution pH where pH is a measure of solution acidity. It is also a common ingredient in deodorant because it can help neutralize smelly. Il est en effet traditionnellement fait du pain au bicarbonate. Establishes general principles for the microfilming of printed newspapers for preservation and distribution in libraries and other documentation services.
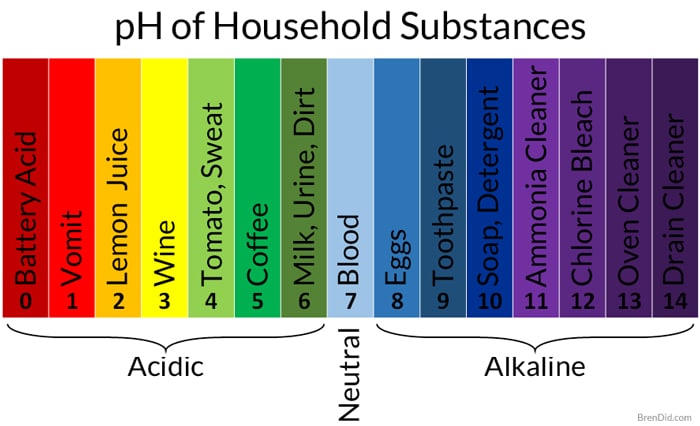 Source: brendid.com
Source: brendid.com
Le bicarbonate de sodium peut être désigné de plusieurs façons selon le contexte ou lépoque. Le bicarbonate de sodium peut être désigné de plusieurs façons selon le contexte ou lépoque. In toothpaste sodium bicarbonate helps to remove stains from teeth by dislodging tiny particles of food or beverages that can blemish tooth enamel. Establishes general principles for the microfilming of printed newspapers for preservation and distribution in libraries and other documentation services. Bicarbonate ions and carbonic acid are present in the blood in a 201 ratio if the blood pH is within the normal range.
 Source: topblogtenz.com
Source: topblogtenz.com
Its a salt with both acidic and basic properties but most importantly it acts as a chemical buffer. To take advantage of the. Bicarbonate de soude carbonate acide de sodium ancien nom sodium bicarbonate médecine NaHCO 3 sodium hydrogen carbonate chimie carbonic acid monosodium salt bicarbonate of soda baking soda ou bread soda anglo-saxon. This is very important in your bloodstream because to maintain cellular. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában.
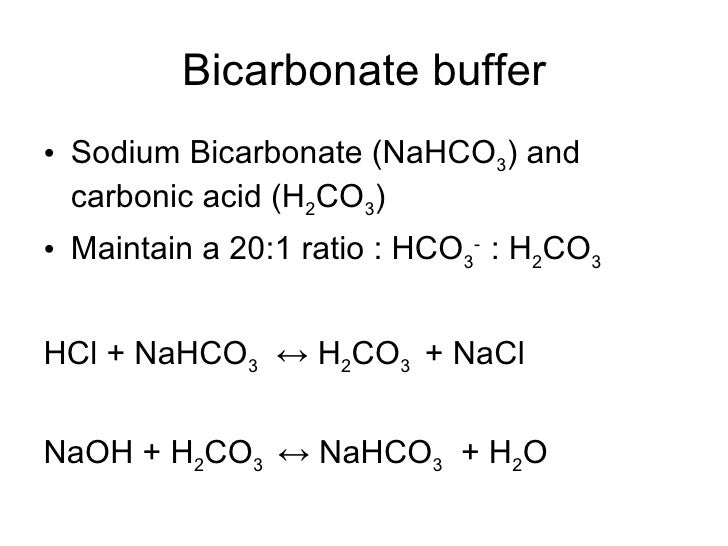 Source: pt.slideshare.net
Source: pt.slideshare.net
Sodium bicarbonate strong acid weak acid salt H 2 CO 3 NaOHHCO 3- H 2 O weak acid strong basebicarbonate water As with the phosphate buffer a weak acid or weak base captures the free ions and a significant change in pH is prevented. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában. Alkalinity affects gluten production in kneaded doughs and also improves browning by reducing the temperature at which the Maillard reaction occurs. Sodium bicarbonate has the chemical formula NaHCO3. KS ISO 40871991 Micrographics - Microfilming of newspapers for archival purposes on 35 mm microfilm.

KS ISO 40871991 Micrographics - Microfilming of newspapers for archival purposes on 35 mm microfilm. Sodium bicarbonate has the chemical formula NaHCO3. In sum sodium bicarbonate should be reserved for severe cases of acidosis only pH. Sodium carbonate has several uses in cuisine largely because it is a stronger base than baking soda sodium bicarbonate but weaker than lye which may refer to sodium hydroxide or less commonly potassium hydroxide. Establishes general principles for the microfilming of printed newspapers for preservation and distribution in libraries and other documentation services.
 Source: youtube.com
Source: youtube.com
Bicarbonate ions and carbonic acid are present in the blood in a 201 ratio if the blood pH is within the normal range. Sodium Bicarbonate mixed with Hydrochloric acid. In toothpaste sodium bicarbonate helps to remove stains from teeth by dislodging tiny particles of food or beverages that can blemish tooth enamel. Establishes general principles for the microfilming of printed newspapers for preservation and distribution in libraries and other documentation services. Sodium carbonate has several uses in cuisine largely because it is a stronger base than baking soda sodium bicarbonate but weaker than lye which may refer to sodium hydroxide or less commonly potassium hydroxide.
 Source: topblogtenz.com
Source: topblogtenz.com
In sum sodium bicarbonate should be reserved for severe cases of acidosis only pH. Sodium bicarbonate has the chemical formula NaHCO3. In sum sodium bicarbonate should be reserved for severe cases of acidosis only pH. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában. To take advantage of the.
 Source: wwwchem.uwimona.edu.jm
Source: wwwchem.uwimona.edu.jm
The abrasive texture of baking soda crystals is useful for cleaning dirt and stains from a variety of surfaces including teeth. Sodium bicarbonate is soluble in water and can be separated from water through evaporation. The chemical reaction observed showed that there was fizzing and bubbling this is evidence that a new gas was being produced. In skincare and personal care products like lotions and bath salts sodium bicarbonate helps control a products acid-base balance to keep it from spoiling. The gas released during an acid-base reaction between baking soda and an acid such as cream of tartar lemon juice or lactic acid in buttermilk causes baked goods to rise.
 Source: en.wikipedia.org
Source: en.wikipedia.org
We would like to show you a description here but the site wont allow us. In most cases of DKA or severe lactic acidosis the administration of sodium bicarbonate does not decrease mortality even when the acidosis is severe. We would like to show you a description here but the site wont allow us. Sodium bicarbonate is soluble in water and can be separated from water through evaporation. Sodium bicarbonate has the chemical formula NaHCO3.

Its a salt with both acidic and basic properties but most importantly it acts as a chemical buffer. Il est en effet traditionnellement fait du pain au bicarbonate. After the fizzing stopped a liquid was leftover leading me to conclude the liquid leftover leading me to conclude the liquid leftover was the NaCl and H2O 4. To take advantage of the. Bicarbonate ions and carbonic acid are present in the blood in a 201 ratio if the blood pH is within the normal range.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium bicarbonate acid or base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.