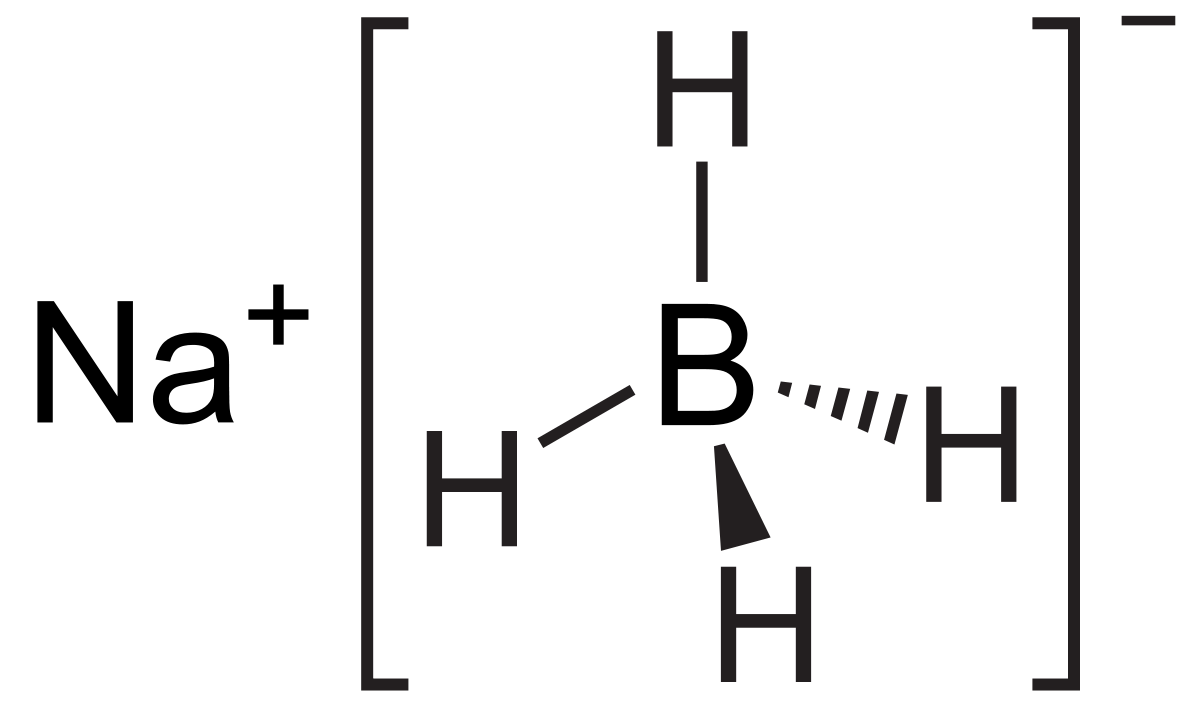
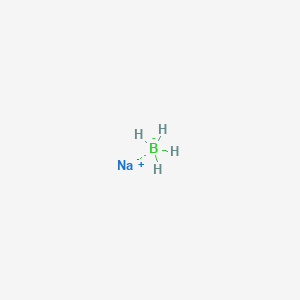
Sodium borohydride molar mass
Sodium Borohydride Molar Mass. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. Sodium borohydride is a white to grayish crystalline powder. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. Sodium borohydride also known as sodium tetrahydridoborate and sodium tetrahydroborate is an inorganic compound with the formula Na BH 4This white solid usually encountered as a powder is a reducing agent that finds application in chemistry both in the laboratory and on an industrial scale.
 Sodium Borohydride Wikipedia From en.wikipedia.org
Sodium Borohydride Wikipedia From en.wikipedia.org
ALL YOUR PAPER NEEDS COVERED 247. Melting Point of sodium chloride. 3 규소 젤 데시칸트 grade 42 43 44silica gel desiccant grade 42 43 44 규소 표준 용액 100 - 01 mgl as sio2silica standard solution 100 - 01 mgl a 규소 1 시약silica 1 reagent 61790-53-2 규조토diatomaceous earth 16893-85-9 규화불화 나트륨sodium silicofluoride. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. It is used in fire extinguishers. Structure of Sodium Chloride.
Mole Concept Molar Mass And Percentage Composition.
MS mass spectrometry. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. Structure of Sodium Chloride. No matter what kind of academic paper you need it is simple and affordable to place your order with Achiever Essays. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Density of sodium chloride.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Molecular Weight Molar Mass of sodium chloride. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Structure of Sodium Chloride. 3 규소 젤 데시칸트 grade 42 43 44silica gel desiccant grade 42 43 44 규소 표준 용액 100 - 01 mgl as sio2silica standard solution 100 - 01 mgl a 규소 1 시약silica 1 reagent 61790-53-2 규조토diatomaceous earth 16893-85-9 규화불화 나트륨sodium silicofluoride. Its only stable isotope is 23 Na.
No matter what kind of academic paper you need it is simple and affordable to place your order with Achiever Essays. B CO 2 -TPD spectra of TiO 2 -400 PdTiO 2 -400 CuTiO 2 -400 and Pd 1 Cu 1 TiO 2 -400. Sodium borohydride is a white to grayish crystalline powder. Sodium borohydride also known as sodium tetrahydridoborate and sodium tetrahydroborate is an inorganic compound with the formula Na BH 4This white solid usually encountered as a powder is a reducing agent that finds application in chemistry both in the laboratory and on an industrial scale. Sodium borohydride NaBH 4 is so stable in water that a 12 aqueous solution stabilized with sodium hydroxide is sold commercially.
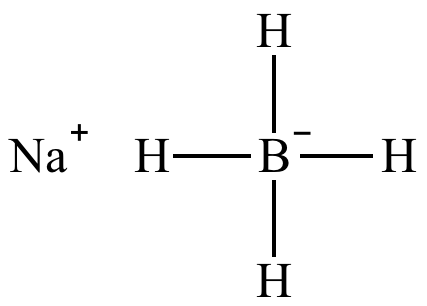 Source: chem.ucla.edu
Source: chem.ucla.edu
It has been tested as pretreatment for pulping of wood but is too costly to be commercialized. MS mass spectrometry. R-BN is a tri-planar structure of BN having ABC stacking in which the formation enthalpy is minimized at relatively low temperature and pressure 1900 K and 01 GPa respectively In h-BN rotation of each basal plane 180 around. No matter what kind of academic paper you need it is simple and affordable to place your order with Achiever Essays. Boiling Point of sodium chloride.
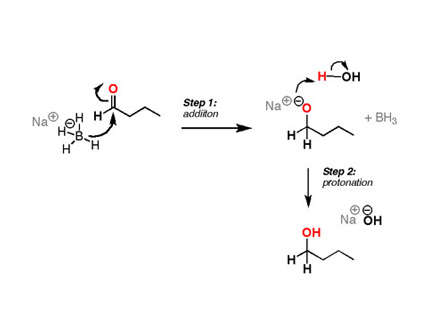 Source: sodiumborohydride.co.uk
Source: sodiumborohydride.co.uk
The free metal does not occur. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Structure of Sodium Chloride NaCl. ALL YOUR PAPER NEEDS COVERED 247. 3 규소 젤 데시칸트 grade 42 43 44silica gel desiccant grade 42 43 44 규소 표준 용액 100 - 01 mgl as sio2silica standard solution 100 - 01 mgl a 규소 1 시약silica 1 reagent 61790-53-2 규조토diatomaceous earth 16893-85-9 규화불화 나트륨sodium silicofluoride.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Molar Mass of Frequently Calculated Chemicals. It has a role as an amphiprotic solvent a member of greenhouse gas a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite and a mouse metabolite. Sodium borohydride also known as sodium tetrahydridoborate and sodium tetrahydroborate is an inorganic compound with the formula Na BH 4This white solid usually encountered as a powder is a reducing agent that finds application in chemistry both in the laboratory and on an industrial scale. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. Molecular Weight Determination Using Solution Colligative Properties.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
R-BN is a tri-planar structure of BN having ABC stacking in which the formation enthalpy is minimized at relatively low temperature and pressure 1900 K and 01 GPa respectively In h-BN rotation of each basal plane 180 around. It has been tested as pretreatment for pulping of wood but is too costly to be commercialized. Density of sodium chloride. Its only stable isotope is 23 Na. C Competitive chemisorption of N 2 red and CO 2 blue on TiO 2.
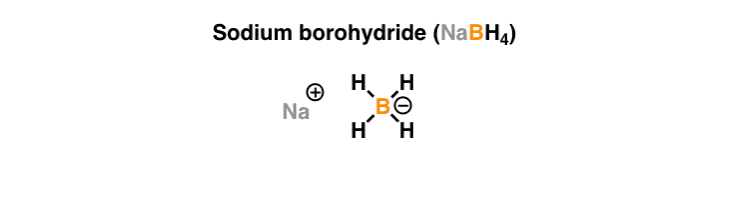 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Molar Mass of Frequently Calculated Chemicals. 3 규소 젤 데시칸트 grade 42 43 44silica gel desiccant grade 42 43 44 규소 표준 용액 100 - 01 mgl as sio2silica standard solution 100 - 01 mgl a 규소 1 시약silica 1 reagent 61790-53-2 규조토diatomaceous earth 16893-85-9 규화불화 나트륨sodium silicofluoride. Its only stable isotope is 23 Na. The free metal does not occur. It is a soft silvery-white highly reactive metalSodium is an alkali metal being in group 1 of the periodic table.
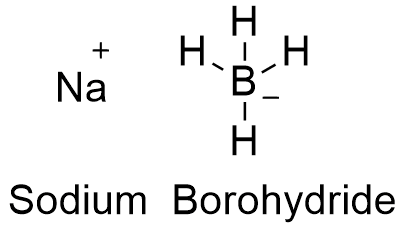 Source: softschools.com
Source: softschools.com
Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. Its only stable isotope is 23 Na. Uses of Sodium Chloride NaCl It is used in medicine Saline solution in nasal spray. In order to effect decomposition the solid or aqueous solution is added to enough water to make the borohydride concentration less than 3 and then excess equivalents of dilute aqueous acetic acid are added drop wise with stirring under nitrogen. R-BN is a tri-planar structure of BN having ABC stacking in which the formation enthalpy is minimized at relatively low temperature and pressure 1900 K and 01 GPa respectively In h-BN rotation of each basal plane 180 around.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Density of sodium chloride. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. It is a soft silvery-white highly reactive metalSodium is an alkali metal being in group 1 of the periodic table. Molecular Weight Determination Using Solution Colligative Properties. It has been tested as pretreatment for pulping of wood but is too costly to be commercialized.
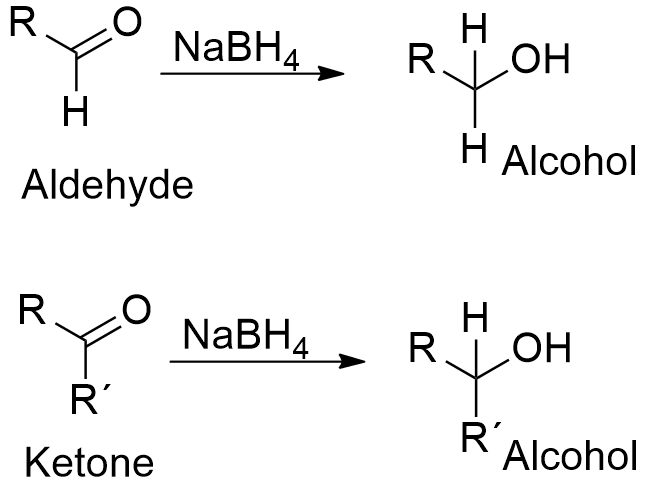 Source: softschools.com
Source: softschools.com
Density of sodium chloride. 3 규소 젤 데시칸트 grade 42 43 44silica gel desiccant grade 42 43 44 규소 표준 용액 100 - 01 mgl as sio2silica standard solution 100 - 01 mgl a 규소 1 시약silica 1 reagent 61790-53-2 규조토diatomaceous earth 16893-85-9 규화불화 나트륨sodium silicofluoride. Structure of Sodium Chloride. Structure of Sodium Chloride NaCl. It is a soft silvery-white highly reactive metalSodium is an alkali metal being in group 1 of the periodic table.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium borohydride molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.