Sodium cyanide strong base
Sodium Cyanide Strong Base. What happens to the pH value when sodium hypochlorite is added to water. Due to the presence of caustic soda in sodium hypo chlorite the. B-H acid 1 base 1 base 2 acid 2 Structurally related acid-base pairs such as H-A and A. HCl can only be a good proton donor however if the Cl -ion is a poor proton acceptor.
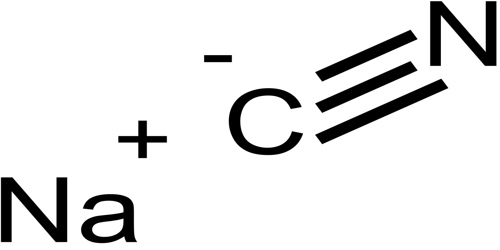 Sodium Cyanide Assignment Point From assignmentpoint.com
Sodium Cyanide Assignment Point From assignmentpoint.com
These characteristics must be kept in mind during transport storage and use of sodium hypochlorite. Due to the presence of caustic soda in sodium hypo chlorite the. HClg H 2 Ol H 3 O aq Cl-aq Strong acid. A good base is usually a good nucleophile. The excretion of 14C-labeled cyanide in rats exposed to chronic intake of potassium cyanide was studied in rats exposed to daily intake of labeled potassium cyanide in the diet for 6 weeks. Lets consider the.
In an acid-base reaction each side of the equilibrium has an acid and a base reactant or product and these may be neutral species or ions.
HCl can only be a good proton donor however if the Cl -ion is a poor proton acceptor. Hypochlorite solutions liberate toxic gases such as chlorine when acidified or heated. The scab never forms so the base can just. In an acid-base reaction each side of the equilibrium has an acid and a base reactant or product and these may be neutral species or ions. HCl is a strong acid. Sodium hypochlorite solution is a weak base that is inflammable.
 Source: en.wikipedia.org
Source: en.wikipedia.org
According to the Brønsted theory an acid is a proton donor and a base is a proton acceptor. RO OH RLi RCC and NH₂. In an acid-base reaction each side of the equilibrium has an acid and a base reactant or product and these may be neutral species or ions. 2 NaOH Cl 2 NaCl NaOCl H 2 O. And B-H are.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The scab never forms so the base can just. If HCl is a strong acid it must be a good proton donor. Describe how older age may compromise the acid-base balance processes. With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. Sodium hypochlorite is a strong oxidator and reacts with flammable compounds and reductors.

These characteristics must be kept in mind during transport storage and use of sodium hypochlorite. Sodium hypochlorite solution is a weak base that is inflammable. What happens to the pH value when sodium hypochlorite is added to water. Acids precipitate proteins which form a protective scab over unharmed tissue but strong bases like sodium hydroxide saponify fatty acids and destroy cell membranes. According to the Brønsted theory an acid is a proton donor and a base is a proton acceptor.
 Source: msrblog.com
Source: msrblog.com
And B-H are. Strong bases have a weak conjugate acid. Thus the Cl-ion must be a weak base. Lets consider the. The excretion of 14C-labeled cyanide in rats exposed to chronic intake of potassium cyanide was studied in rats exposed to daily intake of labeled potassium cyanide in the diet for 6 weeks.
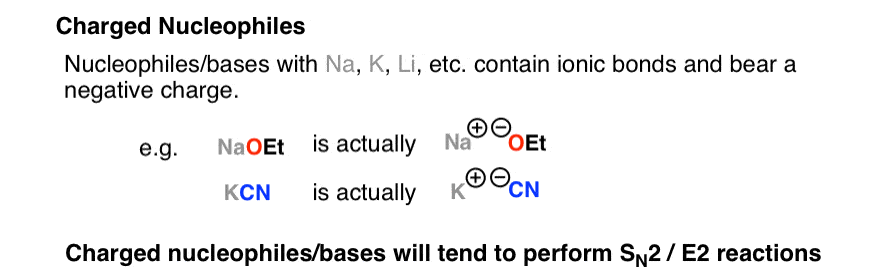 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Included the Solid gas liquid aq The compound potassium cyanide is a. Strong bases have a weak conjugate acid. Its main application in gold mining also exploits its high reactivity toward metalsIt is a moderately strong baseWhen treated with acid it forms the toxic gas hydrogen cyanide. Sodium cyanide is a poisonous compound with the formula Na C NIt is a white water-soluble solid. Urinary excretion was the main route of elimination of cyanide carbon in these rats accounting for 83 of the total excreted radioactivity in 12 hr and 89 of the total excreted radioactivity in 24 hr.
 Source: youtube.com
Source: youtube.com
2 NaOH Cl 2 NaCl NaOCl H 2 O. Strong acids have a weak conjugate base. Acids precipitate proteins which form a protective scab over unharmed tissue but strong bases like sodium hydroxide saponify fatty acids and destroy cell membranes. Sodium hypochlorite is an inexpensive strong oxidizing agent that is used as disinfectant and bleaching agent. If HCl is a strong acid it must be a good proton donor.
 Source: youtube.com
Source: youtube.com
So strong bases substances with negatively charged O N and C atoms are strong nucleophiles. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. HClg H 2 Ol H 3 O aq Cl-aq Strong acid. According to the Brønsted theory an acid is a proton donor and a base is a proton acceptor. Disturbingly its better to be splashed in the eye with concentrated acid than sodium hydroxide.
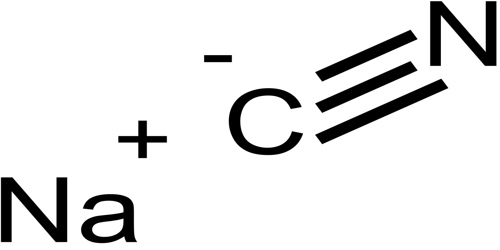 Source: assignmentpoint.com
Source: assignmentpoint.com
Some strong bases are poor nucleophiles because of steric hindrance. Sodium hypochlorite solution is a weak base that is inflammable. Included the Solid gas liquid aq The compound potassium cyanide is a. Due to the presence of caustic soda in sodium hypo chlorite the. What happens to the pH value when sodium hypochlorite is added to water.
 Source: topblogtenz.com
Source: topblogtenz.com
Acids precipitate proteins which form a protective scab over unharmed tissue but strong bases like sodium hydroxide saponify fatty acids and destroy cell membranes. Sodium hypochlorite solution is a weak base that is inflammable. Describe how older age may compromise the acid-base balance processes. It is unstable as a solid but solutions of up to 40 are commercially available that contain NaOH and NaCl as byproducts of the preparation. Sodium chloride is the salt most responsible.
 Source: topblogtenz.com
Source: topblogtenz.com
RO OH RLi RCC and NH₂. The scab never forms so the base can just. Thus the Cl-ion must be a weak base. Urinary excretion was the main route of elimination of cyanide carbon in these rats accounting for 83 of the total excreted radioactivity in 12 hr and 89 of the total excreted radioactivity in 24 hr. RO OH RLi RCC and NH₂.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium cyanide strong base by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.