Sodium hydrogen carbonate solubility in water
Sodium Hydrogen Carbonate Solubility In Water. At this point the solubility curve changes slope and the solubility becomes almost independent of temperature. Due to its medicinal properties he named it sal mirabilis miraculous salt. Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOHIts chemistry is well explored. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.
 Solubility Of Sodium Bicarbonate Nahcolite As A Function Of Download Scientific Diagram From researchgate.net
Solubility Of Sodium Bicarbonate Nahcolite As A Function Of Download Scientific Diagram From researchgate.net
In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the hydrate form is known as Glaubers salt. Reaction with air water and hydrogen. It reacts with water to form sodium hydroxide a corrosive material and methyl alcohol a flammable liquidThe heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. Sodium sulfate has unusual solubility characteristics in water. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.
Its solubility in water rises more than tenfold between 0 C and 32384 C where it reaches a maximum of 497 g100 mL.
It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. Due to its medicinal properties he named it sal mirabilis miraculous salt. Reaction with air water and hydrogen. Sodium sulfate has unusual solubility characteristics in water. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
 Source: researchgate.net
Source: researchgate.net
Preparations of Sodium Sulphate. Sodium sulfate has unusual solubility characteristics in water. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the hydrate form is known as Glaubers salt. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH.
 Source: researchgate.net
Source: researchgate.net
It reacts with water to form sodium hydroxide a corrosive material and methyl alcohol a flammable liquidThe heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. Preparations of Sodium Sulphate. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. Due to its medicinal properties he named it sal mirabilis miraculous salt. It reacts with water to form sodium hydroxide a corrosive material and methyl alcohol a flammable liquidThe heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts.
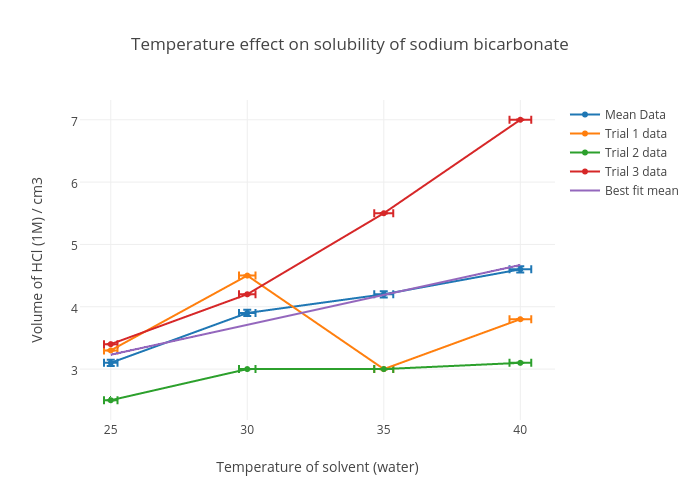 Source: chart-studio.plotly.com
Source: chart-studio.plotly.com
Sodium methylate is a white amorphous powder. It is used to process edible fats and oils and to make other chemicals. Due to its medicinal properties he named it sal mirabilis miraculous salt. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air.
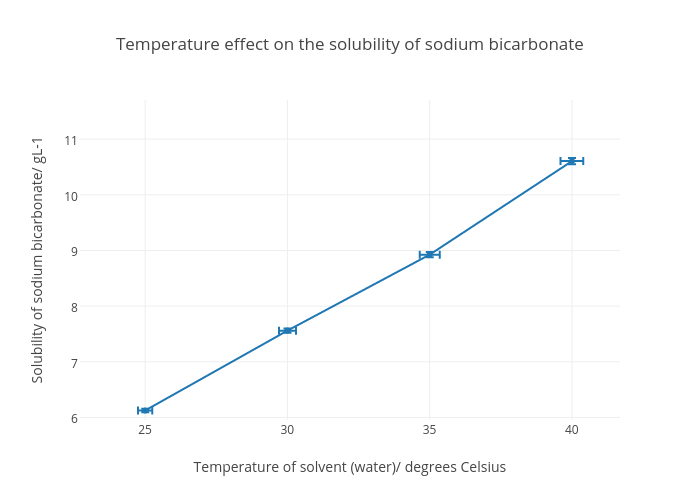 Source: chart-studio.plotly.com
Source: chart-studio.plotly.com
Sodium methylate is a white amorphous powder. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. At this point the solubility curve changes slope and the solubility becomes almost independent of temperature. Sodium sulfate has unusual solubility characteristics in water. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.

Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. It is used to process edible fats and oils and to make other chemicals. Reaction with air water and hydrogen. Preparations of Sodium Sulphate.
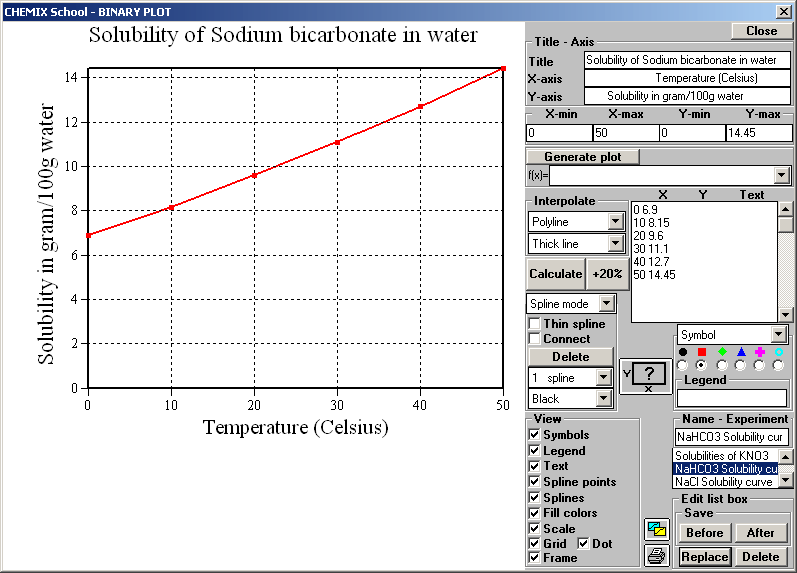 Source: chemix-chemistry-software.com
Source: chemix-chemistry-software.com
One third of the worlds sodium sulfate is produced as by. Sodium is ordinarily quite reactive with air and the reactivity is a function of the relative humidity or water-vapour content of the air. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Due to its medicinal properties he named it sal mirabilis miraculous salt. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the hydrate form is known as Glaubers salt.
 Source: youtube.com
Source: youtube.com
It is used to process edible fats and oils and to make other chemicals. Reaction with air water and hydrogen. Due to its medicinal properties he named it sal mirabilis miraculous salt. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. Preparations of Sodium Sulphate.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
Preparations of Sodium Sulphate. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. It reacts with water to form sodium hydroxide a corrosive material and methyl alcohol a flammable liquidThe heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. Its solubility in water rises more than tenfold between 0 C and 32384 C where it reaches a maximum of 497 g100 mL. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH.
 Source: academia.edu
Source: academia.edu
Sodium methylate is a white amorphous powder. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the hydrate form is known as Glaubers salt. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. At this point the solubility curve changes slope and the solubility becomes almost independent of temperature. It is used to process edible fats and oils and to make other chemicals.
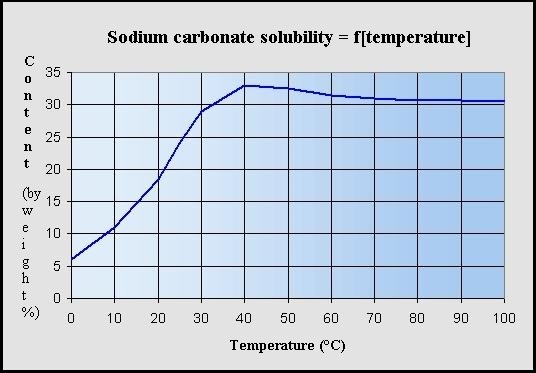 Source: hydro-land.com
Source: hydro-land.com
Generally elemental sodium is more reactive than lithium and it reacts with water to form a strong base sodium hydroxide NaOHIts chemistry is well explored. Sodium sulfate has unusual solubility characteristics in water. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the hydrate form is known as Glaubers salt. Its solubility in water rises more than tenfold between 0 C and 32384 C where it reaches a maximum of 497 g100 mL. Sodium methylate is a white amorphous powder.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium hydrogen carbonate solubility in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.