Sodium hydroxide solubility in water
Sodium Hydroxide Solubility In Water. Sodium hydroxide solution NaOHaq IRRITANT at concentration used see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085. SODIUM HYDROXIDE Caustic Soda is a strong base. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca 2It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with waterIt has many names including hydrated lime caustic lime builders lime slacked lime cal and pickling limeCalcium hydroxide is used in many applications. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas.
 Question 50bb0 Example From socratic.org
Question 50bb0 Example From socratic.org
SODIUM HYDROXIDE Caustic Soda is a strong base. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. Vapor ignites at room temperature. The concentration of the solution does not need to be made up to a high degree of accuracy but should be reasonably close to the same concentration as the dilute hydrochloric acid and less than 05 M. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringentbitter taste. Water 7732 -18 -5 Not applicable Sodium Hydroxide 1310 -73 -2 ACGIH ACGIH Ceiling mgm³ 2 mgm³ OSHA OSHA PEL TWA mgm³ 2 mgm³ IDLH US IDLH mgm³ 10 mgm³ NIOSH NIOSH REL ceiling mgm³ 2 mgm³ 82.
The ignition temperature of sodium in air depends on the area of surface exposed.
Appropriate engineering controls Appropriate engineering controls. The concentration of the solution does not need to be made up to a high degree of accuracy but should be reasonably close to the same concentration as the dilute hydrochloric acid and less than 05 M. Appropriate engineering controls Appropriate engineering controls. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringentbitter taste. Limewater is the common name for a dilute aqueous solution of calcium hydroxideCalcium hydroxide CaOH 2 is sparsely soluble at room temperature in water 15 gL at 25 C. Catalyzes the polymerization of acetaldehyde and other polymerizable compounds.
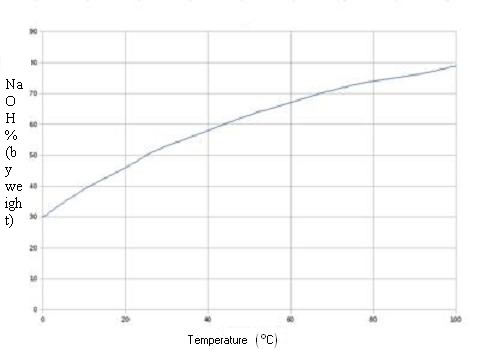 Source: hydro-land.com
Source: hydro-land.com
An agitated pool at 400F. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca 2It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with waterIt has many names including hydrated lime caustic lime builders lime slacked lime cal and pickling limeCalcium hydroxide is used in many applications. Sodium hydroxide solution NaOHaq IRRITANT at concentration used see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. Appropriate engineering controls Appropriate engineering controls.
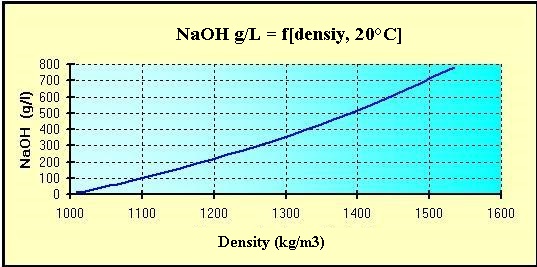 Source: hydro-land.com
Source: hydro-land.com
Emergency eye wash fountains should be available in the immediate vicinity of any potential exposure. The ignition temperature of sodium in air depends on the area of surface exposed. SODIUM HYDROXIDE Caustic Soda is a strong base. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. Droplets at about 250F.
Source:
Reacts rapidly and exothermically with acids both organic and inorganic. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca 2It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with waterIt has many names including hydrated lime caustic lime builders lime slacked lime cal and pickling limeCalcium hydroxide is used in many applications. Reacts rapidly and exothermically with acids both organic and inorganic. Reacts violently with water to give sodium hydroxide and hydrogen which ignites spontaneously Merck 11th ed. SODIUM HYDROXIDE Caustic Soda is a strong base.
 Source: youtube.com
Source: youtube.com
Limewater is the common name for a dilute aqueous solution of calcium hydroxideCalcium hydroxide CaOH 2 is sparsely soluble at room temperature in water 15 gL at 25 C. Catalyzes the polymerization of acetaldehyde and other polymerizable compounds. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringentbitter taste. Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. Limewater is the common name for a dilute aqueous solution of calcium hydroxideCalcium hydroxide CaOH 2 is sparsely soluble at room temperature in water 15 gL at 25 C.
 Source: sciencedirect.com
Source: sciencedirect.com
Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. An agitated pool at 400F. Appropriate engineering controls Appropriate engineering controls. Droplets at about 250F. The ignition temperature of sodium in air depends on the area of surface exposed.
 Source: researchgate.net
Source: researchgate.net
Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. Droplets at about 250F. Catalyzes the polymerization of acetaldehyde and other polymerizable compounds. Calcium hydroxide traditionally called slaked lime is an inorganic compound with the chemical formula Ca 2It is a colorless crystal or white powder and is produced when quicklime calcium oxide is mixed or slaked with waterIt has many names including hydrated lime caustic lime builders lime slacked lime cal and pickling limeCalcium hydroxide is used in many applications. Vapor ignites at room temperature.
 Source: socratic.org
Source: socratic.org
Reacts rapidly and exothermically with acids both organic and inorganic. SODIUM HYDROXIDE Caustic Soda is a strong base. Reacts rapidly and exothermically with acids both organic and inorganic. Vapor ignites at room temperature. Less than or fully saturated limewater is clear and colorless with a slight earthy smell and an astringentbitter taste.
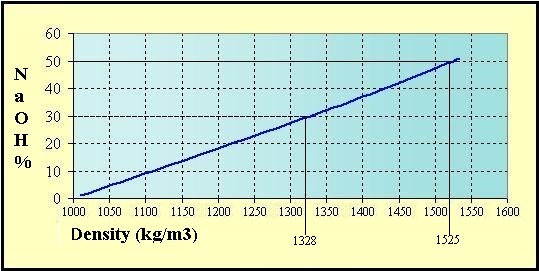 Source: hydro-land.com
Source: hydro-land.com
Reacts rapidly and exothermically with acids both organic and inorganic. An agitated pool at 400F. Droplets at about 250F. The ignition temperature of sodium in air depends on the area of surface exposed. Limewater is the common name for a dilute aqueous solution of calcium hydroxideCalcium hydroxide CaOH 2 is sparsely soluble at room temperature in water 15 gL at 25 C.
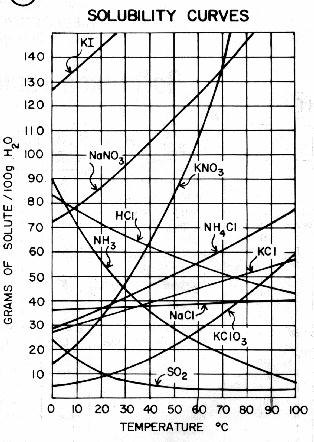 Source: chem.fsu.edu
Source: chem.fsu.edu
The ignition temperature of sodium in air depends on the area of surface exposed. Limewater is the common name for a dilute aqueous solution of calcium hydroxideCalcium hydroxide CaOH 2 is sparsely soluble at room temperature in water 15 gL at 25 C. The concentration of the solution does not need to be made up to a high degree of accuracy but should be reasonably close to the same concentration as the dilute hydrochloric acid and less than 05 M. Reacts violently with water to give sodium hydroxide and hydrogen which ignites spontaneously Merck 11th ed. SODIUM HYDROXIDE Caustic Soda is a strong base.
 Source: researchgate.net
Source: researchgate.net
Catalyzes the polymerization of acetaldehyde and other polymerizable compounds. The ignition temperature of sodium in air depends on the area of surface exposed. Droplets at about 250F. Water 7732 -18 -5 Not applicable Sodium Hydroxide 1310 -73 -2 ACGIH ACGIH Ceiling mgm³ 2 mgm³ OSHA OSHA PEL TWA mgm³ 2 mgm³ IDLH US IDLH mgm³ 10 mgm³ NIOSH NIOSH REL ceiling mgm³ 2 mgm³ 82. SODIUM HYDROXIDE Caustic Soda is a strong base.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium hydroxide solubility in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.