Sodium iodide molecule
Sodium Iodide Molecule. Sodium is an element of group-1. Sodium atoms combine with oxygen to form oxides dioxides and super-oxides. We filmed this short clip on the golden rain reaction to show how beautiful the flakes of lead iodide look in the sun when they precipitate in the cooled down solution. Ph Eur - Find MSDS or SDS a COA data sheets and more information.
 B8a71493 Sodium Iodide 100g Findel Education From findel-education.co.uk
B8a71493 Sodium Iodide 100g Findel Education From findel-education.co.uk
Sodium atoms combine with oxygen to form oxides dioxides and super-oxides. Sodium chloride CAS 7647-14-5 for analysis EMSURE ACSISOReag. 4Na O 2 heat 2Na 2 O. Lead Iodide Golden Rain experiment video. 25 M sodium or potassium iodide 02-30 M sodium thiocyanate 01 M Tris-acetate with 20 M NaCl pH 77 Denaturing 2-6 M guanidineHCl also counts as chaotropic 2-8 M urea also counts as chaotropic 10 M ammonium thiocyanate 1 sodium deoxycholate 1 SDS Organic 10 dioxane 50 ethylene glycol pH 8-115 also counts as chaotropic. We filmed this short clip on the golden rain reaction to show how beautiful the flakes of lead iodide look in the sun when they precipitate in the cooled down solution.
The most common side effects include cough airway problems due to the anaesthesia wearing off reduced blood pressure and other complications such as changes in heart rate.
Lead Iodide Golden Rain experiment video. If you are doing a lab report here is an example. Sodium carbonate can be used if a sulfide compound is not available. Sodium chloride CAS 7647-14-5 for analysis EMSURE ACSISOReag. Ph Eur - Find MSDS or SDS a COA data sheets and more information. 25 M sodium or potassium iodide 02-30 M sodium thiocyanate 01 M Tris-acetate with 20 M NaCl pH 77 Denaturing 2-6 M guanidineHCl also counts as chaotropic 2-8 M urea also counts as chaotropic 10 M ammonium thiocyanate 1 sodium deoxycholate 1 SDS Organic 10 dioxane 50 ethylene glycol pH 8-115 also counts as chaotropic.
 Source: fearschemistry.wikidot.com
Source: fearschemistry.wikidot.com
The video was taken in a dark garage with sunlight coming. Sodium atoms combine with oxygen to form oxides dioxides and super-oxides. If you are doing a lab report here is an example. Sodium is an element of group-1. Sodium carbonate can be used if a sulfide compound is not available.
Therefore the tendency of sodium atoms to bind to oxygen is much higher. Sodium carbonate can be used if a sulfide compound is not available. Sodium is an element of group-1. Sodium hydride is the chemical compound with the empirical formula Na HThis alkali metal hydride is primarily used as a strong yet combustible base in organic synthesisNaH is a saline salt-like hydride composed of Na and H ions in contrast to molecular hydrides such as borane methane ammonia and waterIt is an ionic material that is insoluble in organic solvents although. Therefore the tendency of sodium atoms to bind to oxygen is much higher.
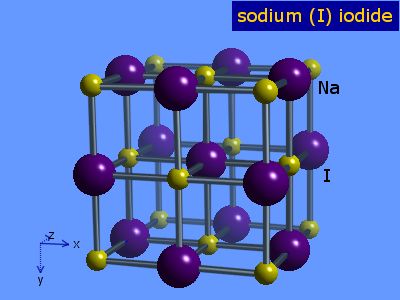 Source: webelements.com
Source: webelements.com
Sodium hydride is the chemical compound with the empirical formula Na HThis alkali metal hydride is primarily used as a strong yet combustible base in organic synthesisNaH is a saline salt-like hydride composed of Na and H ions in contrast to molecular hydrides such as borane methane ammonia and waterIt is an ionic material that is insoluble in organic solvents although. 4Na O 2 heat 2Na 2 O. If you are doing a lab report here is an example. Sodium chloride CAS 7647-14-5 for analysis EMSURE ACSISOReag. Lead Iodide Golden Rain experiment video.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium hydride is the chemical compound with the empirical formula Na HThis alkali metal hydride is primarily used as a strong yet combustible base in organic synthesisNaH is a saline salt-like hydride composed of Na and H ions in contrast to molecular hydrides such as borane methane ammonia and waterIt is an ionic material that is insoluble in organic solvents although. We filmed this short clip on the golden rain reaction to show how beautiful the flakes of lead iodide look in the sun when they precipitate in the cooled down solution. Sodium hydride is the chemical compound with the empirical formula Na HThis alkali metal hydride is primarily used as a strong yet combustible base in organic synthesisNaH is a saline salt-like hydride composed of Na and H ions in contrast to molecular hydrides such as borane methane ammonia and waterIt is an ionic material that is insoluble in organic solvents although. Sodium atoms combine with oxygen to form oxides dioxides and super-oxides. Sodium is an element of group-1.
 Source: byjus.com
Source: byjus.com
We filmed this short clip on the golden rain reaction to show how beautiful the flakes of lead iodide look in the sun when they precipitate in the cooled down solution. Sodium chloride CAS 7647-14-5 for analysis EMSURE ACSISOReag. 2Na I 2 2NaI sodium iodide The reaction of sodiumNa atoms with oxygen. If you are doing a lab report here is an example. Sodium is an element of group-1.
Source: chemspider.com
Sugammadex sold under the brand name Bridion is a medication for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in general anaesthesiaIt is the first selective relaxant binding agent SRBA. Sodium carbonate can be used if a sulfide compound is not available. 2Na I 2 2NaI sodium iodide The reaction of sodiumNa atoms with oxygen. Sodium chloride CAS 7647-14-5 for analysis EMSURE ACSISOReag. The most common side effects include cough airway problems due to the anaesthesia wearing off reduced blood pressure and other complications such as changes in heart rate.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
25 M sodium or potassium iodide 02-30 M sodium thiocyanate 01 M Tris-acetate with 20 M NaCl pH 77 Denaturing 2-6 M guanidineHCl also counts as chaotropic 2-8 M urea also counts as chaotropic 10 M ammonium thiocyanate 1 sodium deoxycholate 1 SDS Organic 10 dioxane 50 ethylene glycol pH 8-115 also counts as chaotropic. Sugammadex sold under the brand name Bridion is a medication for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in general anaesthesiaIt is the first selective relaxant binding agent SRBA. We filmed this short clip on the golden rain reaction to show how beautiful the flakes of lead iodide look in the sun when they precipitate in the cooled down solution. Lead Iodide Golden Rain experiment video. Sodium hydride is the chemical compound with the empirical formula Na HThis alkali metal hydride is primarily used as a strong yet combustible base in organic synthesisNaH is a saline salt-like hydride composed of Na and H ions in contrast to molecular hydrides such as borane methane ammonia and waterIt is an ionic material that is insoluble in organic solvents although.
 Source: findel-education.co.uk
Source: findel-education.co.uk
25 M sodium or potassium iodide 02-30 M sodium thiocyanate 01 M Tris-acetate with 20 M NaCl pH 77 Denaturing 2-6 M guanidineHCl also counts as chaotropic 2-8 M urea also counts as chaotropic 10 M ammonium thiocyanate 1 sodium deoxycholate 1 SDS Organic 10 dioxane 50 ethylene glycol pH 8-115 also counts as chaotropic. If you are doing a lab report here is an example. Sodium is an element of group-1. Sodium carbonate can be used if a sulfide compound is not available. Lead Iodide Golden Rain experiment video.
 Source: fishersci.fi
Source: fishersci.fi
Sodium atoms combine with oxygen to form oxides dioxides and super-oxides. Ph Eur - Find MSDS or SDS a COA data sheets and more information. The video was taken in a dark garage with sunlight coming. Sodium hydride is the chemical compound with the empirical formula Na HThis alkali metal hydride is primarily used as a strong yet combustible base in organic synthesisNaH is a saline salt-like hydride composed of Na and H ions in contrast to molecular hydrides such as borane methane ammonia and waterIt is an ionic material that is insoluble in organic solvents although. The most common side effects include cough airway problems due to the anaesthesia wearing off reduced blood pressure and other complications such as changes in heart rate.
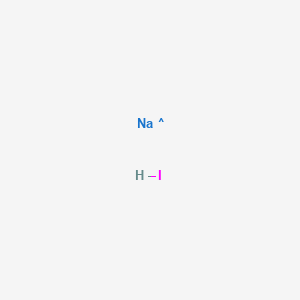
If you are doing a lab report here is an example. 4Na O 2 heat 2Na 2 O. Ph Eur - Find MSDS or SDS a COA data sheets and more information. Sodium atoms combine with oxygen to form oxides dioxides and super-oxides. Therefore the tendency of sodium atoms to bind to oxygen is much higher.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium iodide molecule by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.