Sodium iodide solubility
Sodium Iodide Solubility. Solubility changes that follow chemical change are important in ecology. 5844 gmol Chemical Formula. Precipitates of carbonate ion colours. It is used mainly as a nutritional supplement and in organic chemistry.
 Is Nai Soluble Or Insoluble In Water Youtube From youtube.com
Is Nai Soluble Or Insoluble In Water Youtube From youtube.com
Solubility of carbonates have a variation because there are soluble and insoluble carbonates. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the hydrate form is known as Glaubers salt. When iodide and iodine meet at waterchloroform-hexane surfaces the triiodide ion formed dissolves in the water not the non-polar solvent mixture. Personal Protective Equipment Eyes. When the solution cools beautiful lead iodide crystals will fall out of solution. Preparations of Sodium Sulphate.
Personal Protective Equipment Eyes.
In general solids become more. It is used mainly as a nutritional supplement and in organic chemistry. 5844 gmol Chemical Formula. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate. Hydrogen iodide and insoluble in ethanol. Personal Protective Equipment Eyes.
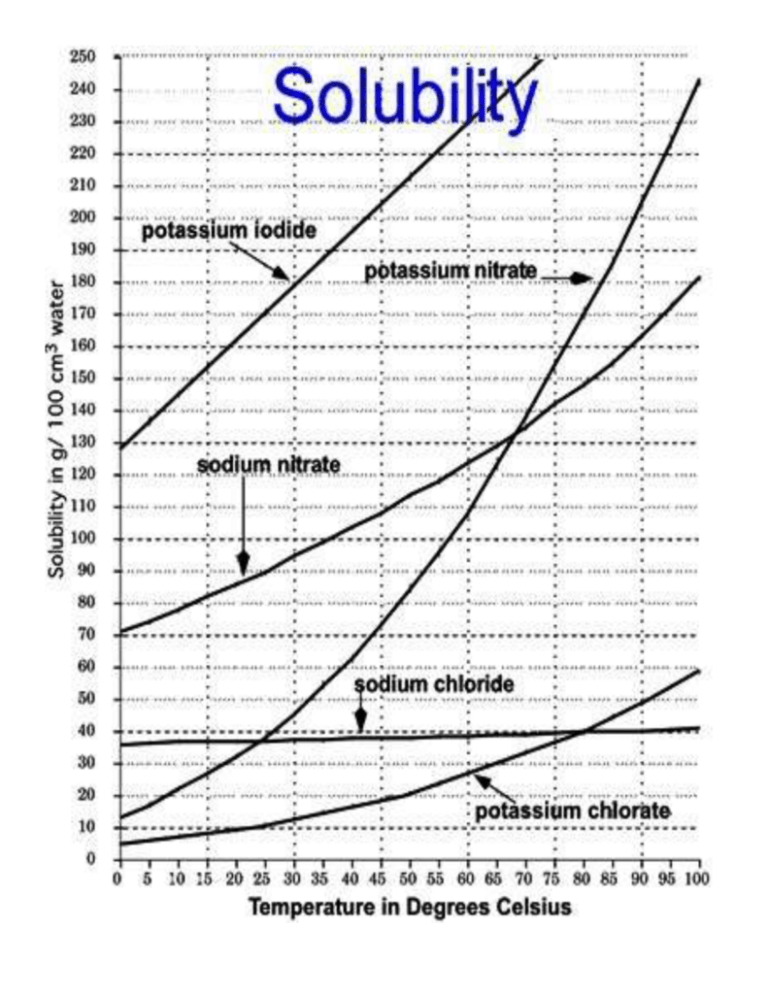 Source: studylib.net
Source: studylib.net
Although non-polar molecular iodine cannot dissolve in water it reacts with iodide ion to form something that can. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate. Now we consider about those precipitate of anions and those precipitates colours. 106404 View Pricing Availability. Hydrogen iodide and insoluble in ethanol.
 Source: youtube.com
Source: youtube.com
Its solubility in water rises more than tenfold between 0 C and 32384 C where it reaches a maximum of 497 g100 mL. Due to its medicinal properties he named it sal mirabilis miraculous salt. A chemical change may make. Personal Protective Equipment Eyes. It is produced industrially as the salt formed.

Although it is not itself flammable the solid product and even 30 solutions in water are powerful oxidizing agents. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. Lead iodide golden rain experiment requirements. Sodium chlorate appears as an odorless pale yellow to white crystalline solid. But when we study deeply about solubility of.
 Source: researchgate.net
Source: researchgate.net
106404 View Pricing Availability. 1790 GL 20C Specific GravityDensity36670gcm3 Molecular FormulaINa Molecular Weight14989 Section 10 - Stability and Reactivity Chemical Stability. Stoichiometric amounts of lead nitrate and potassium iodide are combined with enough water to dissolve all of the lead iodide precipitate at 80 degrees Celsius. Carbonate sulfate sulphite phosphate sulfide chloride bromide iodide and more anions form precipitates with some metal ions. Personal Protective Equipment Eyes.
 Source: en.wikipedia.org
Source: en.wikipedia.org
1790 GL 20C Specific GravityDensity36670gcm3 Molecular FormulaINa Molecular Weight14989 Section 10 - Stability and Reactivity Chemical Stability. No OSHA Vacated PELs are listed for this chemical. The golden rain reaction takes advantage of the increased solubility of lead iodide in hot water. In general about 3 mL of the solvent is used with 01 g or 02 mL 2 - 3 drops of the substance. Radiolabelled compound DB09293 is used as a diagnostic tool to evaluate thyroid function and morphology.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The effect of temperature. Hydrogen iodide and insoluble in ethanol. It is appreciably soluble in water and heavier so may be expected to sink and dissolve at a rapid rate. When iodide and iodine meet at waterchloroform-hexane surfaces the triiodide ion formed dissolves in the water not the non-polar solvent mixture. Contact with wood organic matter ammonium salts sulfur sulfuric acid various metals and.
 Source: sodium.atomistry.com
Source: sodium.atomistry.com
Solubility changes that follow chemical change are important in ecology. It is produced industrially as the salt formed. 106404 View Pricing Availability. Its solubility in water rises more than tenfold between 0 C and 32384 C where it reaches a maximum of 497 g100 mL. In general about 3 mL of the solvent is used with 01 g or 02 mL 2 - 3 drops of the substance.
 Source: web.tenafly.k12.nj.us
Source: web.tenafly.k12.nj.us
The class of compound may be indicated from the following table. Under standard conditions it is a white water-soluble solid comprising a 11 mix of sodium cations Na and iodide anions I in a crystal lattice. Although it is not itself flammable the solid product and even 30 solutions in water are powerful oxidizing agents. Sodium iodide is a water-soluble ionic compound with a crystal latticeSodium iodide is a source of iodine and can be administered as a supplement for total parenteral nutrition but is more commonly used in veterinary medicine. 1790 GL 20C Specific GravityDensity36670gcm3 Molecular FormulaINa Molecular Weight14989 Section 10 - Stability and Reactivity Chemical Stability.

When iodide and iodine meet at waterchloroform-hexane surfaces the triiodide ion formed dissolves in the water not the non-polar solvent mixture. This is our newest publication and has been created to support the school technician profession in Scotland. But when we study deeply about solubility of. You can see from the balanced equation there is a 21 mole ratio between silver iodide and sodium sulfide. Now we consider about those precipitate of anions and those precipitates colours.
 Source: researchgate.net
Source: researchgate.net
You can see from the balanced equation there is a 21 mole ratio between silver iodide and sodium sulfide. Contact with wood organic matter ammonium salts sulfur sulfuric acid various metals and. Now we consider about those precipitate of anions and those precipitates colours. Stoichiometric amounts of lead nitrate and potassium iodide are combined with enough water to dissolve all of the lead iodide precipitate at 80 degrees Celsius. In 1625 Johann Rudolf Glauber discovered the sodium sulfate from Austrian spring water there so the hydrate form is known as Glaubers salt.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium iodide solubility by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






