Sodium oxide decomposes
Sodium Oxide Decomposes. Used for making gasoline additives electric power cable sodium lamps other chemicals. Anhydrous sodium dithionite decomposes to sodium sulfate and sulfur dioxide above 90 C in the air. Sodium borohydride is a white to grayish crystalline powder. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited.
 Chemical Equations And Reactions Describing Chemical Reactions Ppt Download From slideplayer.com
Chemical Equations And Reactions Describing Chemical Reactions Ppt Download From slideplayer.com
It is used in Kodak. Sodium hydride is a compound which decomposes but does not melt at 752 F 400 C. Reaction is given below 3NaOCl aq 2NaCl aq NaClO 3aq It is used in oxidation of starch. Oxidation of metals Sodium hypochlorite reacts with metals and forms metal oxide by oxidizing them. Reaction of sodium with hydrogen at a temperature of above 392 F 200 C results in the formation of sodium hydride NaH. Sodium borohydride is a white to grayish crystalline powder.
Sodium hydride is a compound which decomposes but does not melt at 752 F 400 C.
Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130200 C a process that generates sodium oxide which in a. If this burning process is carried out under pressure sodium superoxide NaO 2 is formed. Burns violently with explosions that may spatter the material. Shipped as a solid or molten liquid. It decomposes at high temperature and forms sodium chlorate and sodium chloride. Oxidation of metals Sodium hypochlorite reacts with metals and forms metal oxide by oxidizing them.
 Source: youtube.com
Source: youtube.com
It decomposes at high temperature and forms sodium chlorate and sodium chloride. The octahydrate is produced by treating sodium hydroxide with hydrogen peroxide. Anhydrous sodium dithionite decomposes to sodium sulfate and sulfur dioxide above 90 C in the air. Reaction is given below NaOCl Mg MgO NaCl. Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130200 C a process that generates sodium oxide which in a.
 Source: slideplayer.com
Source: slideplayer.com
Sodium appears as a silvery soft metal that becomes grayish white upon exposure to air. Reaction is given below 3NaOCl aq 2NaCl aq NaClO 3aq It is used in oxidation of starch. Sodium hydride is a compound which decomposes but does not melt at 752 F 400 C. Reaction is given below NaOCl Mg MgO NaCl. Sodium borohydride is a white to grayish crystalline powder.
 Source: chegg.com
Source: chegg.com
In absence of air it decomposes quickly. The octahydrate is produced by treating sodium hydroxide with hydrogen peroxide. With further heating above the 657 C boiling point the compound decomposes to Na 2 O releasing O 2. Whereas on burning in limited supply of oxygen it forms sodium oxide Na 2 O. Sodium appears as a silvery soft metal that becomes grayish white upon exposure to air.
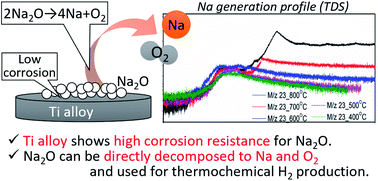
2 Na 2 O 2 2 Na 2 O O 2 Preparation. Oxidation of metals Sodium hypochlorite reacts with metals and forms metal oxide by oxidizing them. Reaction is given below NaOCl Mg MgO NaCl. It is used in Kodak. Burns violently with explosions that may spatter the material.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Reaction of sodium with hydrogen at a temperature of above 392 F 200 C results in the formation of sodium hydride NaH. Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130200 C a process that generates sodium oxide which in a. Reaction is given below 3NaOCl aq 2NaCl aq NaClO 3aq It is used in oxidation of starch. Reaction of sodium with hydrogen at a temperature of above 392 F 200 C results in the formation of sodium hydride NaH. Whereas on burning in limited supply of oxygen it forms sodium oxide Na 2 O.
 Source: slideplayer.com
Source: slideplayer.com
It is used in Kodak. Oxidation of metals Sodium hypochlorite reacts with metals and forms metal oxide by oxidizing them. Sodium borohydride is a white to grayish crystalline powder. Reaction of sodium with hydrogen at a temperature of above 392 F 200 C results in the formation of sodium hydride NaH. Anhydrous sodium dithionite decomposes to sodium sulfate and sulfur dioxide above 90 C in the air.
 Source: slideplayer.com
Source: slideplayer.com
Whereas on burning in limited supply of oxygen it forms sodium oxide Na 2 O. It is used in Kodak. The octahydrate is produced by treating sodium hydroxide with hydrogen peroxide. Burns violently with explosions that may spatter the material. Sodium borohydride is a white to grayish crystalline powder.
 Source: youtube.com
Source: youtube.com
2 Na 2 O 2 2 Na 2 O O 2 Preparation. If this burning process is carried out under pressure sodium superoxide NaO 2 is formed. 2 Na 2 O 2 2 Na 2 O O 2 Preparation. Burns violently with explosions that may spatter the material. Pyrithione can be prepared in a two-step synthesis from 2-bromopyridine by oxidation to the N-oxide with a suitable peracid followed by substitution using sodium dithionite to introduce the thiol functional group.
 Source: slideplayer.com
Source: slideplayer.com
Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130200 C a process that generates sodium oxide which in a. Pyrithione can be prepared in a two-step synthesis from 2-bromopyridine by oxidation to the N-oxide with a suitable peracid followed by substitution using sodium dithionite to introduce the thiol functional group. It is decomposed by water to form sodium hydroxide a corrosive material and hydrogen a flammable gasThe heat of this reaction may be sufficient to ignite the hydrogenThe material itself is easily ignited and burns vigorously once ignited. It is used in Kodak. Used for making gasoline additives electric power cable sodium lamps other chemicals.
 Source: studylib.net
Source: studylib.net
Reaction is given below 3NaOCl aq 2NaCl aq NaClO 3aq It is used in oxidation of starch. Sodium borohydride is a white to grayish crystalline powder. Sodium peroxide can be prepared on a large scale by the reaction of metallic sodium with oxygen at 130200 C a process that generates sodium oxide which in a. If this burning process is carried out under pressure sodium superoxide NaO 2 is formed. It is used in Kodak.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium oxide decomposes by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.