Sodium oxide reaction with water
Sodium Oxide Reaction With Water. Neutralisation is a reaction between an acid and an alkali that forms a salt and water. It also serves as a ligand in coordination chemistry. Pehrsson et al 2008. The molecule is planar.
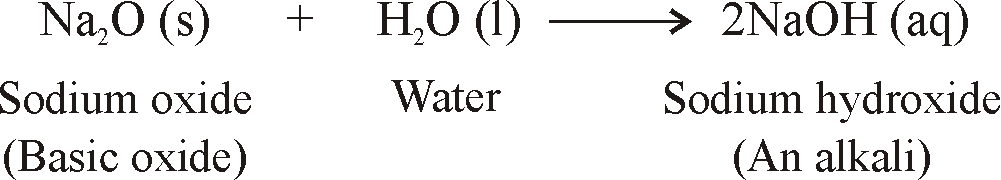 Chemical Properties Of Metals Tutormate From tutormate.in
Chemical Properties Of Metals Tutormate From tutormate.in
An amine oxide also known as an amine N-oxide and N-oxide is a chemical compound that contains the functional group R 3 N O an NO coordinate covalent bond with three additional hydrogen andor hydrocarbon side chains attached to N. Water is relatively low in sodium but sodium levels vary by water source and with the use of water-softening systems Bradshaw and Powell 2002. Neutralisation is a reaction between an acid and an alkali that forms a salt and water. Tea and coffee are also very low in sodium although the level may increase slightly with the. The color is created by the reaction of nitric oxide formed from sodium nitrite with myoglobin to form nitric oxide myoglobin. Pyridine-N-oxide is the heterocyclic compound with the formula C 5 H 5 NOThis colourless hygroscopic solid is the product of the oxidation of pyridineIt was originally prepared using peroxyacids as the oxidising agent.
Pehrsson et al 2008.
Pehrsson et al 2008. In the strict sense the term amine oxide applies only to oxides of tertiary amines. Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate. It also serves as a ligand in coordination chemistry. Tea and coffee are also very low in sodium although the level may increase slightly with the. Water is relatively low in sodium but sodium levels vary by water source and with the use of water-softening systems Bradshaw and Powell 2002.
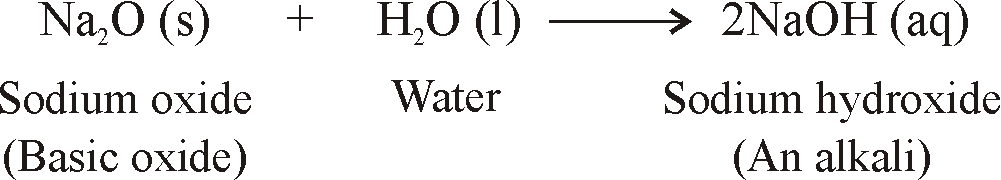 Source: tutormate.in
Source: tutormate.in
Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate. The color is created by the reaction of nitric oxide formed from sodium nitrite with myoglobin to form nitric oxide myoglobin. Water is relatively low in sodium but sodium levels vary by water source and with the use of water-softening systems Bradshaw and Powell 2002. It also serves as a ligand in coordination chemistry. Indicators are substances that change colour with a change in acidityalkalinity.
 Source: beautyofscience.pivotshare.com
Source: beautyofscience.pivotshare.com
An amine oxide also known as an amine N-oxide and N-oxide is a chemical compound that contains the functional group R 3 N O an NO coordinate covalent bond with three additional hydrogen andor hydrocarbon side chains attached to N. Salts are odourless and have a salty taste and many are soluble in water. The compound is used infrequently as an oxidizing reagent in organic synthesis. The molecule is planar. In the strict sense the term amine oxide applies only to oxides of tertiary amines.
 Source: fliphtml5.com
Source: fliphtml5.com
Neutralisation is a reaction between an acid and an alkali that forms a salt and water. Water is relatively low in sodium but sodium levels vary by water source and with the use of water-softening systems Bradshaw and Powell 2002. The molecule is planar. Pyridine-N-oxide is the heterocyclic compound with the formula C 5 H 5 NOThis colourless hygroscopic solid is the product of the oxidation of pyridineIt was originally prepared using peroxyacids as the oxidising agent. Neutralisation is a reaction between an acid and an alkali that forms a salt and water.
 Source: youtube.com
Source: youtube.com
It also serves as a ligand in coordination chemistry. Indicators are substances that change colour with a change in acidityalkalinity. The pH scale is used to measure acidity and alkalinity. Pyridine-N-oxide is the heterocyclic compound with the formula C 5 H 5 NOThis colourless hygroscopic solid is the product of the oxidation of pyridineIt was originally prepared using peroxyacids as the oxidising agent. In the strict sense the term amine oxide applies only to oxides of tertiary amines.
 Source: toppr.com
Source: toppr.com
Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate. Sometimes it is written as R 3 NO or wrongly as R 3 NO. Pyridine-N-oxide is the heterocyclic compound with the formula C 5 H 5 NOThis colourless hygroscopic solid is the product of the oxidation of pyridineIt was originally prepared using peroxyacids as the oxidising agent. Tea and coffee are also very low in sodium although the level may increase slightly with the. In the strict sense the term amine oxide applies only to oxides of tertiary amines.
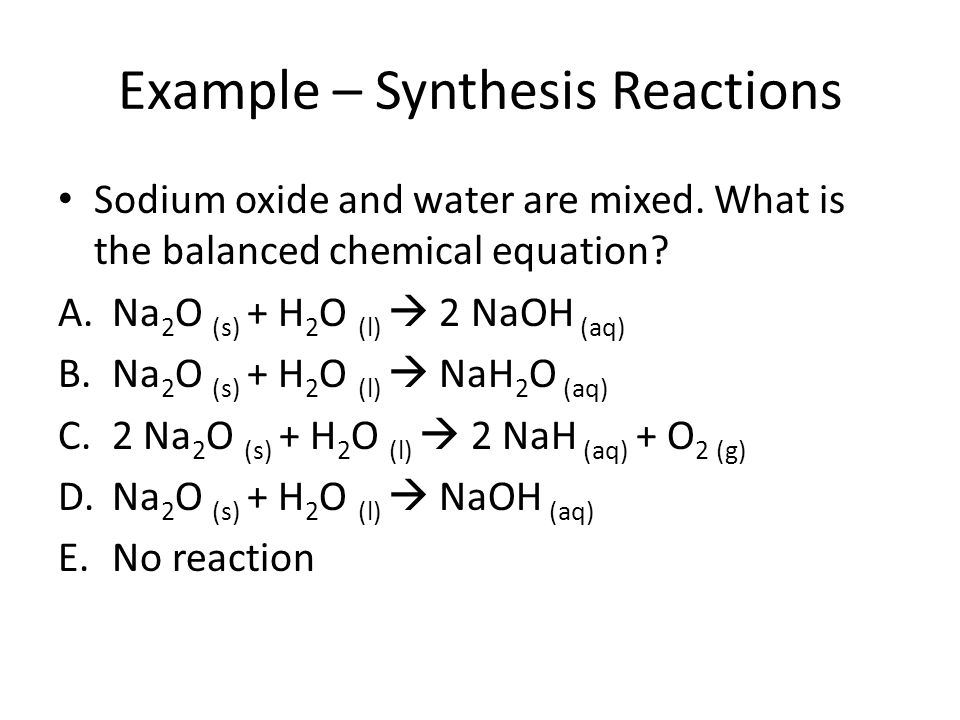 Source: slideplayer.com
Source: slideplayer.com
The molecule is planar. The pH scale is used to measure acidity and alkalinity. It also serves as a ligand in coordination chemistry. Pyridine-N-oxide is the heterocyclic compound with the formula C 5 H 5 NOThis colourless hygroscopic solid is the product of the oxidation of pyridineIt was originally prepared using peroxyacids as the oxidising agent. Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate.
 Source: youtube.com
Source: youtube.com
The molecule is planar. The color is created by the reaction of nitric oxide formed from sodium nitrite with myoglobin to form nitric oxide myoglobin. The compound is used infrequently as an oxidizing reagent in organic synthesis. The molecule is planar. It also serves as a ligand in coordination chemistry.
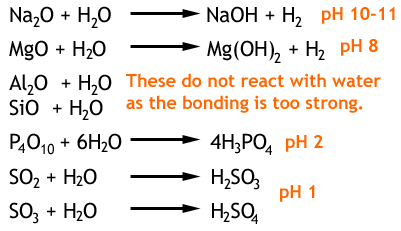 Source: scienceaid.net
Source: scienceaid.net
An amine oxide also known as an amine N-oxide and N-oxide is a chemical compound that contains the functional group R 3 N O an NO coordinate covalent bond with three additional hydrogen andor hydrocarbon side chains attached to N. Indicators are substances that change colour with a change in acidityalkalinity. Pyridine-N-oxide is the heterocyclic compound with the formula C 5 H 5 NOThis colourless hygroscopic solid is the product of the oxidation of pyridineIt was originally prepared using peroxyacids as the oxidising agent. Salts are odourless and have a salty taste and many are soluble in water. Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate.
 Source: youtube.com
Source: youtube.com
An amine oxide also known as an amine N-oxide and N-oxide is a chemical compound that contains the functional group R 3 N O an NO coordinate covalent bond with three additional hydrogen andor hydrocarbon side chains attached to N. Neutralisation is a reaction between an acid and an alkali that forms a salt and water. An amine oxide also known as an amine N-oxide and N-oxide is a chemical compound that contains the functional group R 3 N O an NO coordinate covalent bond with three additional hydrogen andor hydrocarbon side chains attached to N. Pyridine-N-oxide is the heterocyclic compound with the formula C 5 H 5 NOThis colourless hygroscopic solid is the product of the oxidation of pyridineIt was originally prepared using peroxyacids as the oxidising agent. Salts are odourless and have a salty taste and many are soluble in water.
 Source: study.com
Source: study.com
Water is relatively low in sodium but sodium levels vary by water source and with the use of water-softening systems Bradshaw and Powell 2002. Water is relatively low in sodium but sodium levels vary by water source and with the use of water-softening systems Bradshaw and Powell 2002. The molecule is planar. Pehrsson et al 2008. Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sodium oxide reaction with water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.