Sodium perchlorate chemical formula
Sodium Perchlorate Chemical Formula. The simplest name iron chloride will in this case be. Chemical formula Synonyms CAS Number DBr. CHAPTER 3 Chemical Equations Reaction Stoichiometry. K2SO4 potassium sulfate 59.
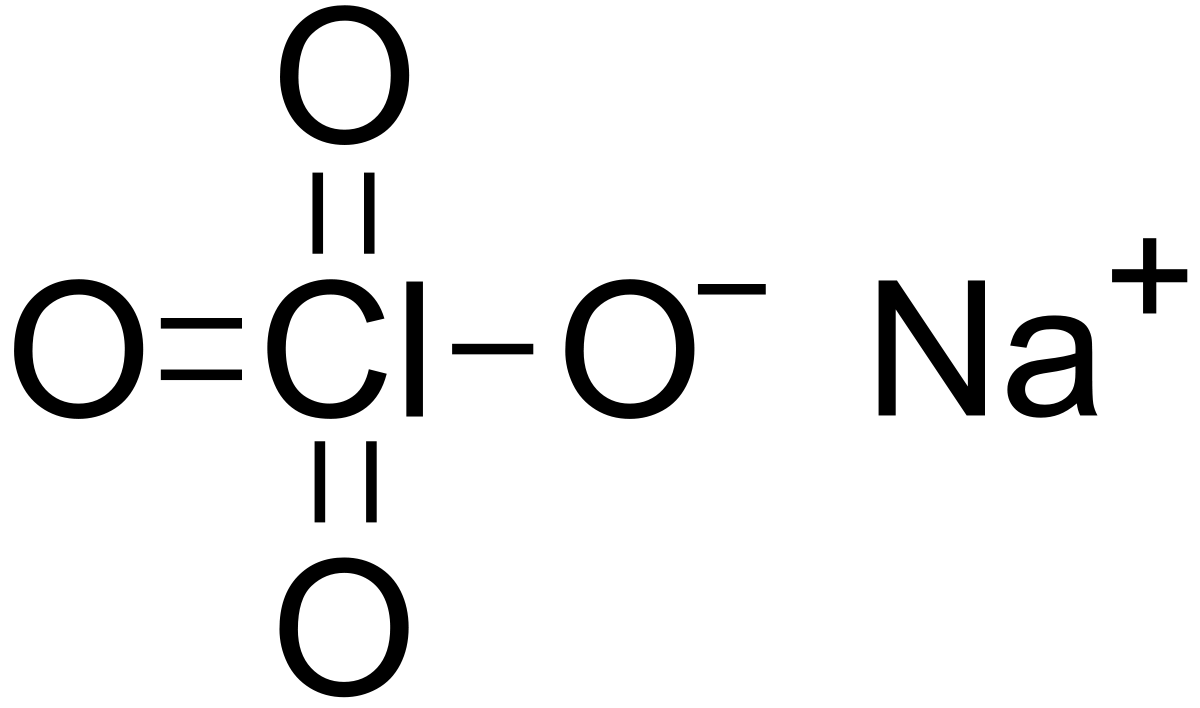 Sodium Perchlorate Wikipedia From en.wikipedia.org
Sodium Perchlorate Wikipedia From en.wikipedia.org
Sodium perchlorate appears as white crystalline solid. If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. This process is the main outlet for the industrial production of perchloric acid. CHAPTER 3 Chemical Equations Reaction Stoichiometry. An explosion occurred during heating of a mixture of potassium chlorate and magnesium Chem. CID 5360545 Sodium CID 19654 Chloric acid Dates.
Sodium chlorate appears as an odorless pale yellow to white crystalline solid.
A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában. Co2SO43 cobalt III sulfate 60. Hg2Cl2 mercury I chloride 57. Skill 3-3 Mass Percent and the Chemical Formula 12 The formula shows the number of moles of each element. Because the charges on the ions. NaMnO4 sodium permanganate 50.
 Source: youtube.com
Source: youtube.com
Mg3N2 magnesium nitride 49. KMnO4 potassium permanganate Write the chemical formula for each of the following ionic compounds. The nomenclature of binary covalent compounds follows these rules. An explosion occurred during heating of a mixture of potassium chlorate and magnesium Chem. An ionic compound is never formed between two cations only or two anions only.

The information in a chemical formula. An explosion occurred during heating of a mixture of potassium chlorate and magnesium Chem. Chemical compound - chemical compound - Binary molecular covalent compounds. The salt also can be produced by salt metathesis reaction of ammonium salts with sodium perchlorate. The key to writing proper ionic formulas is simple.
 Source: oneclass.com
Source: oneclass.com
Chemical compound - chemical compound - Binary molecular covalent compounds. Prolonged exposure to fire or heat may result in an explosion. 3 Objectives Understand how to write chemical equations Perform calculations based on chemical equations Calculate percent yields from chemical reactions Understand the concept of sequential reactions. Use it to calculate the mass percent of each element on a mole basis. The formula of the sugar glucose is C 6 H 12 O.
 Source: en.wikipedia.org
Source: en.wikipedia.org
If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. 3 Objectives Understand how to write chemical equations Perform calculations based on chemical equations Calculate percent yields from chemical reactions Understand the concept of sequential reactions. Use it to calculate the mass percent of each element on a mole basis. NaCIO4 sodium perchlorate 47. The charge of the metal ion is determined from the formula of the compound and the charge of the anion.
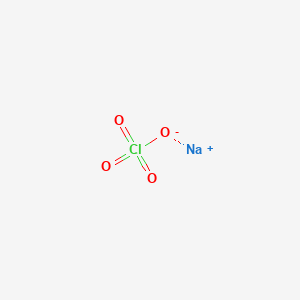
Although there are no ions in these compounds they are named in a similar manner to binary ionic compounds. If large quantities are involved in a fire or the combustible material is finely divided an explosion may result. CHAPTER 3 Chemical Equations Reaction Stoichiometry. An ionic compound is never formed between two cations only or two anions only. How many moles and formulas are in 416 g ammonium carbonate.
 Source: youtube.com
Source: youtube.com
The formula of the sugar glucose is C 6 H 12 O. How many moles and formulas are in 416 g ammonium carbonate. CID 5360545 Sodium CID 19654 Chloric acid Dates. Prolonged exposure to fire or heat may result in an explosion. If large quantities are involved in a fire or the combustible material is finely divided an explosion may result.
 Source: chegg.com
Source: chegg.com
This process is the main outlet for the industrial production of perchloric acid. Noncombustible but will accelerate the burning of combustible materials. For example consider binary ionic compounds of iron and chlorine. The salt also can be produced by salt metathesis reaction of ammonium salts with sodium perchlorate. A mixture of finely divided magnesium and nitric acid is explosive Pieters.

Sodium chlorate appears as an odorless pale yellow to white crystalline solid. Use it to calculate the mass percent of each element on a mole basis. An ionic compound is never formed between two cations only or two anions only. A mixture of finely divided magnesium and nitric acid is explosive Pieters. A Google ingyenes szolgáltatása azonnal lefordítja a szavakat kifejezéseket és weboldalakat a magyar és több mint 100 további nyelv kombinációjában.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
An ionic compound is never formed between two cations only or two anions only. Chemical formulas for ionic compounds are called ionic formulas The chemical formula for an ionic compound. The key to writing proper ionic formulas is simple. Because the charges on the ions. The charge of the metal ion is determined from the formula of the compound and the charge of the anion.
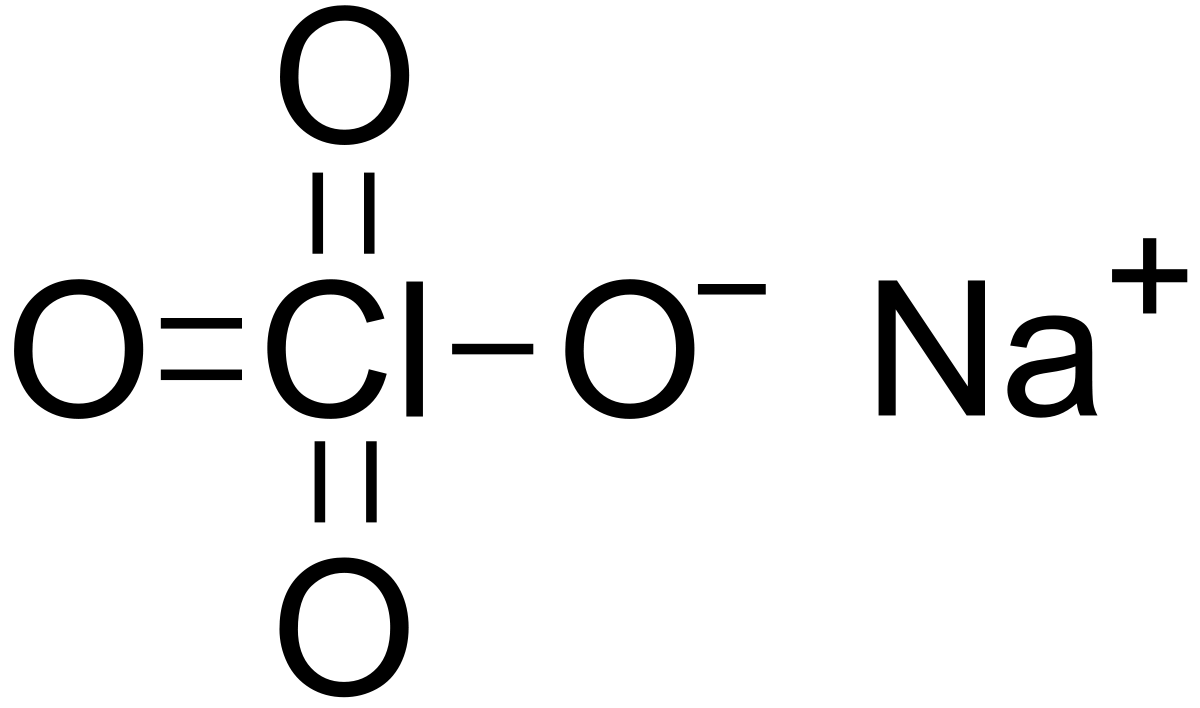 Source: en.wikipedia.org
Source: en.wikipedia.org
Because the charges on the ions. Prolonged exposure to fire or heat may result in an explosion. A mixture of finely divided magnesium and nitric acid is explosive Pieters. NaMnO4 sodium permanganate 50. This process is the main outlet for the industrial production of perchloric acid.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium perchlorate chemical formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.