Sodium phosphate salt equation
Sodium Phosphate Salt Equation. In this reaction phosphoric acid H_3PO_4 a weak acid will react with calcium hydroxide CaOH_2 a strong base to produce calcium phosphate Ca_3PO_4_2 an insoluble salt and water. Double displacement reactions that feature a carbonate reacting with an acid have the net ionic equation2 H CO 3 2 – H 2 O CO 2 If every ion is a spectator ion then there was no reaction and the net ionic equation is null. The correct formula for sodium chloride table salt is. NaCl Na₂Cl NaCl₂ Na₂Cl₂ Correct Wrong.
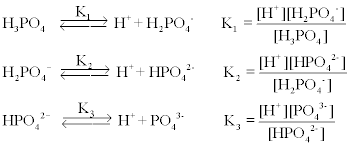 Phosphate Buffer Issues From chem.fsu.edu
Phosphate Buffer Issues From chem.fsu.edu
This reaction produces sodium sulfate carbon dioxide and water. Acid Reactive metal Salt Hydrogen Gas. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Salt of non-Potassium Sodium and Ammonium. Nitric acid has the chemical formula HNO3 and Calcium Hydroxide has the chemical formula CaOH2. Preparation of Zinc.
Nitric acid has the chemical formula HNO3 and Calcium Hydroxide has the chemical formula CaOH2.
The correct formula for sodium chloride table salt is. The first part of the name is sodium so you know youre looking for an element and not a cation. Acid Metal Carbonate Salt Water Carbon Dioxide. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. High-temperature sodiumsulfur batteries operating at 300350 C have been commercially applied for large-scale energy storage and conversion. For example the compound sodium chloride is written NaCl where Na is the cation and Cl-is the anion.
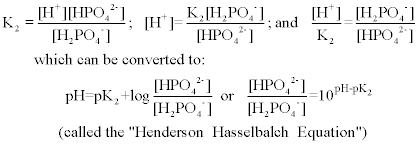 Source: chem.fsu.edu
Source: chem.fsu.edu
This reaction produces sodium sulfate carbon dioxide and water. Prepare 01 M sodium phosphate dibasic. The symbol is Na. For example the compound sodium chloride is written NaCl where Na is the cation and Cl-is the anion. Acid-Base Balance and Disorders.
 Source: youtube.com
Source: youtube.com
H_2SO_4aq 2NaHCO_3aq rarr Na_2SO_4aq. Owing to the Lewis acidity of the Li ions LiPF 6 also. Put 80 mL of sodium phosphate dibasic stock 05 M from Step 1 in a beaker and add H2O to give a final volume of 400 mL. Acid Alkali Salt Water. Acid Reactive metal Salt Hydrogen Gas.
 Source: youtube.com
Source: youtube.com
Prepare 01 M sodium phosphate dibasic. Answer 1 of 13. When an acid and a base react with each other the products that are formed is a salt an ionic compound that is formed from a reaction between an acid and a base and water. The symbol is Na. The correct formula for sodium chloride table salt is.
 Source: breslyn.org
Source: breslyn.org
On a typical western diet an adult generates approximately 081 mEqkg body weight of nonvolatile acid and 15000 mEq of CO 2 volatile acid dailyDepending on the pCO 2 a small fraction of CO 2 is dissolved in body fluids as carbonic acid H 2 CO 3 a weak acid while a large amount of CO 2 is eliminated through respiration. The symbol is Na. When an acid and a base react with each other the products that are formed is a salt an ionic compound that is formed from a reaction between an acid and a base and water. The net electrical charge of an anion is denoted using a superscript after the chemical species symbol. H_2SO_4aq 2NaHCO_3aq rarr Na_2SO_4aq.
There is a reaction between sulfuric acid and sodium bicarbonate also called sodium hydrogen carbonate and baking soda. The net electrical charge of an anion is denoted using a superscript after the chemical species symbol. Preparation of Sodium Chloride NaCl HCl NaOH NaCl H2O. Acid Reactive metal Salt Hydrogen Gas. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.
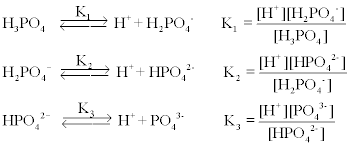 Source: chem.fsu.edu
Source: chem.fsu.edu
Acid Alkali Salt Water. Acid Reactive metal Salt Hydrogen Gas. The general equation for this reaction is. Judging from the products of the reaction. The first part of the name is sodium so you know youre looking for an element and not a cation.
 Source: youtube.com
Source: youtube.com
Double displacement reactions that feature a carbonate reacting with an acid have the net ionic equation2 H CO 3 2 – H 2 O CO 2 If every ion is a spectator ion then there was no reaction and the net ionic equation is null. Acid carbonate rarr salt carbon dioxide water The balanced chemical equation for this reaction is. Acid Reactive metal Salt Hydrogen Gas. Salt of non-Potassium Sodium and Ammonium. Judging from the products of the reaction.
 Source: softschools.com
Source: softschools.com
Since many elements display a range of valences determining the anion and cation in a chemical formula isnt always clear-cut. Acid Metal Oxide Salt Water. Prepare 01 M sodium phosphate dibasic. Answer 1 of 13. High-temperature sodiumsulfur batteries operating at 300350 C have been commercially applied for large-scale energy storage and conversion.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
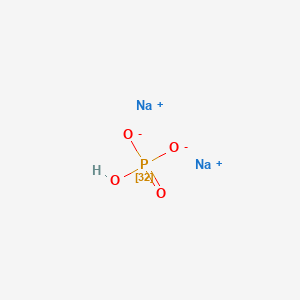
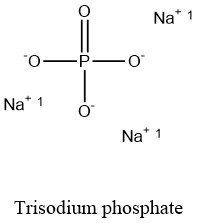
H_2SO_4aq 2NaHCO_3aq rarr Na_2SO_4aq. Owing to the Lewis acidity of the Li ions LiPF 6 also. There is a reaction between sulfuric acid and sodium bicarbonate also called sodium hydrogen carbonate and baking soda. Dissolving sodium dihydrogen phosphate NaH 2PO 4 K a 62 10 8 and sodium hydrogen phosphate Na 2HPO 4 together in an aqueous solution. For example the compound sodium chloride is written NaCl where Na is the cation and Cl-is the anion.

Double displacement reactions that feature a carbonate reacting with an acid have the net ionic equation2 H CO 3 2 – H 2 O CO 2 If every ion is a spectator ion then there was no reaction and the net ionic equation is null. For example the compound sodium chloride is written NaCl where Na is the cation and Cl-is the anion. Double displacement reactions that feature a carbonate reacting with an acid have the net ionic equation2 H CO 3 2 – H 2 O CO 2 If every ion is a spectator ion then there was no reaction and the net ionic equation is null. It hydrolyzes near 70 C 158 F according to the following equation forming highly toxic HF gas. The second part of the name has an -ide ending which means youre dealing with a simple element anion.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium phosphate salt equation by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.