Sodium silicate reacts with
Sodium Silicate Reacts With. PhosphorusV oxide reacts with water to give phosphoric v acid. The nitrogen gas liberated by this process instantly inflates the air bag. It will react with strong bases to form silicate salts. In the presence of aldehydes sodium dithionite reacts either to form α-hydroxy-sulfinates at room temperature or to reduce the aldehyde to the corresponding alcohol above a.
 Acid Triggered Hydrolysis Of Sodium Silicate Na2sio3 And Subsequent Download Scientific Diagram From researchgate.net
Acid Triggered Hydrolysis Of Sodium Silicate Na2sio3 And Subsequent Download Scientific Diagram From researchgate.net
First the sodium reacts with potassium nitrate to produce potassium oxide K2O sodium oxide Na2O and additional nitro-gen gas. Recall from Chapter 1 that the fundamental building block in silicate minerals is an SiO 4 4-tetrahedron with oxygen at the corners and silicon in the center. These reactions exhibit complex pH-dependent equilibria involving bisulfite thiosulfate and sulfur dioxide. Since calcium carbonate is relatively insoluble it tends to come out of solution. Calcium bicarbonate entering with the feed water is broken down at boiler temperatures or reacts with caustic soda to form calcium carbonate. Less electronegative elements tend to form basic oxides such as sodium oxide and magnesium oxide.
Silicate minerals dominate igneous rocks because silicon and oxygen are the most common elements in magmas.
Sodium dithionite reacts with oxygen. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon. Silicon dioxide is the anhydride of silicic acid. Less electronegative elements tend to form basic oxides such as sodium oxide and magnesium oxide. Sodium silicate is used to react selectively with magnesium hardness. P 4 O 6 6 H 2 O 4 H 3 PO 3.
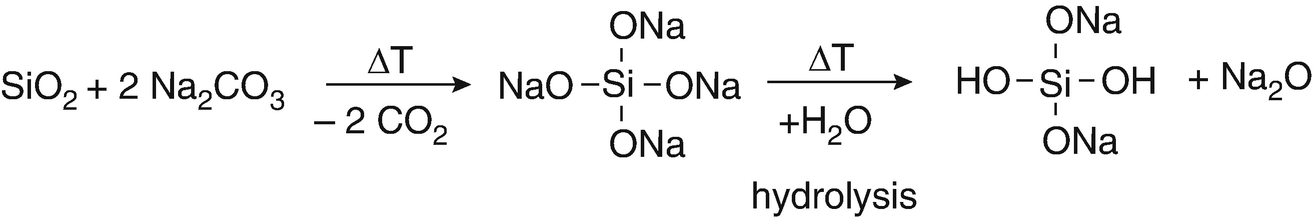 Source: link.springer.com
Source: link.springer.com
Learn more about the characteristics distribution and uses of silicon in this article. Recall from Chapter 1 that the fundamental building block in silicate minerals is an SiO 4 4-tetrahedron with oxygen at the corners and silicon in the center. Silicon dioxide is the anhydride of silicic acid. The metal oxides K2O and Na2O react with silicon dioxide in a final reaction to produce silicate glass which is harmless and stable. Assume that the nitrogen gas liberated.
 Source: sciencedirect.com
Source: sciencedirect.com
Sodium silicate is used to react selectively with magnesium hardness. Assume that the nitrogen gas liberated. Sodium silicate is used to react selectively with magnesium hardness. P 4 O 6 6 H 2 O 4 H 3 PO 3. Learn more about the characteristics distribution and uses of silicon in this article.
 Source: researchgate.net
Source: researchgate.net
Silicate minerals dominate igneous rocks because silicon and oxygen are the most common elements in magmas. Sodium carbonate partially breaks down at high temperature to sodium hydroxide caustic and carbon. Learn more about the characteristics distribution and uses of silicon in this article. Since calcium carbonate is relatively insoluble it tends to come out of solution. In the presence of aldehydes sodium dithionite reacts either to form α-hydroxy-sulfinates at room temperature or to reduce the aldehyde to the corresponding alcohol above a.
 Source: pubs.rsc.org
Source: pubs.rsc.org
Learn more about the characteristics distribution and uses of silicon in this article. Na 2 S 2 O 4 O 2 H 2 O NaHSO 4 NaHSO 3. Sodium silicate is used to react selectively with magnesium hardness. These reactions exhibit complex pH-dependent equilibria involving bisulfite thiosulfate and sulfur dioxide. Silicate minerals dominate igneous rocks because silicon and oxygen are the most common elements in magmas.
 Source: sciencedirect.com
Source: sciencedirect.com
PhosphorusV oxide reacts with water to give phosphoric v acid. PhosphorusIII oxide reacts to form phosphorous acid in water. Calcium bicarbonate entering with the feed water is broken down at boiler temperatures or reacts with caustic soda to form calcium carbonate. It is the second most abundant element in the crust being surpassed only by oxygen. Assume that the nitrogen gas liberated.
 Source: researchgate.net
Source: researchgate.net
First the sodium reacts with potassium nitrate to produce potassium oxide K2O sodium oxide Na2O and additional nitro-gen gas. Recall from Chapter 1 that the fundamental building block in silicate minerals is an SiO 4 4-tetrahedron with oxygen at the corners and silicon in the center. Na 2 S 2 O 4 O 2 H 2 O NaHSO 4 NaHSO 3. In the presence of aldehydes sodium dithionite reacts either to form α-hydroxy-sulfinates at room temperature or to reduce the aldehyde to the corresponding alcohol above a. It will react with strong bases to form silicate salts.
 Source: researchgate.net
Source: researchgate.net
Silicon dioxide is the anhydride of silicic acid. Na 2 S 2 O 4 O 2 H 2 O NaHSO 4 NaHSO 3. The nitrogen gas liberated by this process instantly inflates the air bag. PhosphorusIII oxide reacts to form phosphorous acid in water. Recall from Chapter 1 that the fundamental building block in silicate minerals is an SiO 4 4-tetrahedron with oxygen at the corners and silicon in the center.
 Source: researchgate.net
Source: researchgate.net
First the sodium reacts with potassium nitrate to produce potassium oxide K2O sodium oxide Na2O and additional nitro-gen gas. Silicate minerals dominate igneous rocks because silicon and oxygen are the most common elements in magmas. First the sodium reacts with potassium nitrate to produce potassium oxide K2O sodium oxide Na2O and additional nitro-gen gas. P 4 O 6 6 H 2 O 4 H 3 PO 3. PhosphorusV oxide reacts with water to give phosphoric v acid.
 Source: researchgate.net
Source: researchgate.net
Learn more about the characteristics distribution and uses of silicon in this article. PhosphorusIII oxide reacts to form phosphorous acid in water. Silicon a nonmetallic chemical element in the carbon family that makes up 277 percent of Earths crust. Learn more about the characteristics distribution and uses of silicon in this article. The nitrogen gas liberated by this process instantly inflates the air bag.
 Source: sciencedirect.com
Source: sciencedirect.com
It is the second most abundant element in the crust being surpassed only by oxygen. Silicate minerals dominate igneous rocks because silicon and oxygen are the most common elements in magmas. PhosphorusIII oxide reacts to form phosphorous acid in water. Since calcium carbonate is relatively insoluble it tends to come out of solution. Learn more about the characteristics distribution and uses of silicon in this article.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium silicate reacts with by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.