Sodium sulfide mass
Sodium Sulfide Mass. Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas. Even in the absence of air the reaction may be accompanied by flame Mellor 10132 1946-47. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide. The molar mass of calcium sulfide is 7214 gmol.
 What Is The Formula For Sodium Sulfide Socratic From socratic.org
What Is The Formula For Sodium Sulfide Socratic From socratic.org
It is a soft silvery-white highly reactive metal. Even in the absence of air the reaction may be accompanied by flame Mellor 10132 1946-47. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. They are water-soluble giving strongly alkaline solutions. Sodium sulfide is the chemical compound with the formula Na 2 S or more commonly its hydrate Na 2 S9H 2 OBoth the anhydrous and the hydrated salts are colorless solids. Reacts very vigorously with gaseous hydrogen sulfide.
Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure.
The molar mass of sodium oxide is 6198 gmol. Sodium sulfide anhydrous is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. Even in the absence of air the reaction may be accompanied by flame Mellor 10132 1946-47. The free metal does not occur in nature and must be prepared from compounds. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Sodium sulfide anhydrous is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. Sodium sulfide anhydrous is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. Beef extract 30 g Peptone 300 g Ferrous ammonium sulphat 02 g Sodium thiosulphate 0025 g Agar 30g Final pH at 25C 7302 Distilled water 1000ml. Sodium is an alkali metal being in group 1 of the periodic table because it has a single electron in its outer shell that it. The free metal does not occur in nature and must be prepared from compounds.

Its only stable isotope is 23 Na. The curves are calculated based on the ideal cathodeanode active mass ratio and normalized by the total active mass in both electrodes. Sodium is a soft silvery-white highly reactive metal. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas.
 Source: youtube.com
Source: youtube.com
2Na 1O 2299 gmol 16 gmol 6198 gmol. The molar mass of calcium sulfide is 7214 gmol. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide. Some commercial samples are specified as Na 2 SxH 2 O. Sodium sulfide is the chemical compound with the formula Na 2 S or more commonly its hydrate Na 2 S9H 2 OBoth the anhydrous and the hydrated salts are colorless solids.
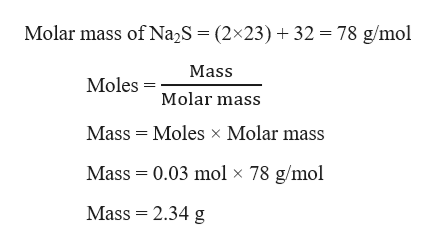 Source: bartleby.com
Source: bartleby.com
Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. The free metal does not occur in nature and must be prepared from compounds. Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas. If exposed to moist air it is liable to spontaneous heating and may cause ignition of nearby combustible material.
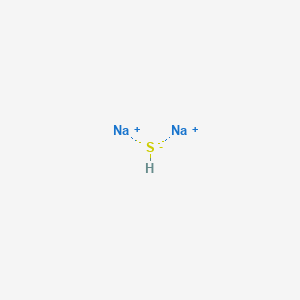
Sodium sulfide anhydrous is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. The free metal does not occur in nature and must be prepared from compounds. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. 2Na 1O 2299 gmol 16 gmol 6198 gmol. Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas.
 Source: en.wikipedia.org
Source: en.wikipedia.org
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide. The curves are calculated based on the ideal cathodeanode active mass ratio and normalized by the total active mass in both electrodes. Sodium is a soft silvery-white highly reactive metal. Sodium is the sixth most abundant element in the Earths crust and exists.
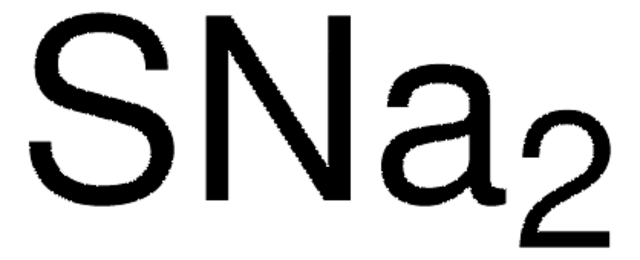 Source: sigmaaldrich.com
Source: sigmaaldrich.com
They are water-soluble giving strongly alkaline solutions. Even in the absence of air the reaction may be accompanied by flame Mellor 10132 1946-47. Sodium is a chemical element with the symbol Na from Latin natrium and atomic number 11. C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide. 2Na 1O 2299 gmol 16 gmol 6198 gmol.
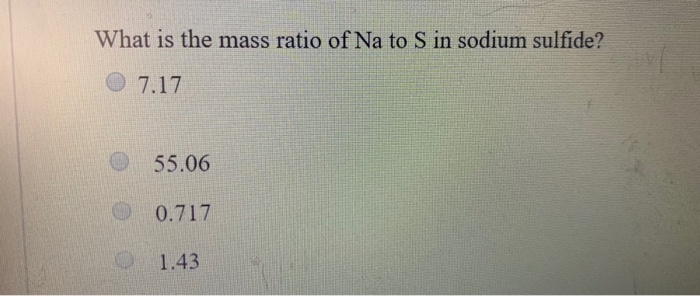 Source: chegg.com
Source: chegg.com
Reacts very vigorously with gaseous hydrogen sulfide. Production of hydrogen sulfide can be detected when ferrous sulfide a black precipitate is produced as a result of ferrous ammonium sulfate reacting with H2S gas. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. The chemical symbol for Sodium is Na. Sodium is an alkali metal being in group 1 of the periodic table because it has a single electron in its outer shell that it.
 Source: socratic.org
Source: socratic.org
They are water-soluble giving strongly alkaline solutions. Its only stable isotope is 23 Na. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. Even in the absence of air the reaction may be accompanied by flame Mellor 10132 1946-47. The molar mass of sodium oxide is 6198 gmol.
 Source: youtube.com
Source: youtube.com
Sodium is a soft silvery-white highly reactive metal. The curves are calculated based on the ideal cathodeanode active mass ratio and normalized by the total active mass in both electrodes. Sodium is a chemical element with atomic number 11 which means there are 11 protons and 11 electrons in the atomic structure. If exposed to moist air it is liable to spontaneous heating and may cause ignition of nearby combustible material. The molar mass of sodium oxide is 6198 gmol.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title sodium sulfide mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.