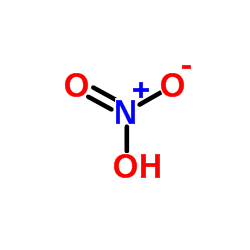
Solid cadmium oxide decomposition formula
Solid Cadmium Oxide Decomposition Formula. Aelectrolysis Bfractional distillation Cincomplete combustion Dthermal decomposition. BCarbon is burnt to provide heat. The researchers used this nano zinc oxide as a curing agent in neoprene rubber. A naturally occurring oxide sourced from ilmenite rutile and anatase it has a wide range of applications.
 The Plots Of The Decomposition Of A Pure Cadmium Oxide Cdo Sample Download Scientific Diagram From researchgate.net
The Plots Of The Decomposition Of A Pure Cadmium Oxide Cdo Sample Download Scientific Diagram From researchgate.net
A short summary of this paper. 28 Full PDFs related to this paper. Microscopic images and surface area studies showed that the prepared zinc oxide had particle sizes in the range 1530 nm and surface area in the range 1230 m 2 g. CIron III oxide is reduced to iron by carbon dioxide. The researchers used this nano zinc oxide as a curing agent in neoprene rubber. DThe raw materials are bauxite limestone and coke.
Residues of calcium oxide are exempted from the requirement of a tolerance when used in accordance with good agricultural practice as inert or occasionally active ingredients in pesticide formulations applied to growing crops or to raw agricultural commodities after harvest.
BCarbon is burnt to provide heat. Manganese dioxide is the inorganic compound with the formula MnO 2This blackish or brown solid occurs naturally as the mineral pyrolusite which is the main ore of manganese and a component of manganese nodulesThe principal use for MnO 2 is for dry-cell batteries such as the alkaline battery and the zinccarbon battery. Metal oxides thus typically contain an anion of oxygen in the oxidation state of 2. CIron III oxide is reduced to iron by carbon dioxide. Titanium dioxide is a titanium oxide with the formula TiO2. Most of the Earths crust consists of solid oxides the result of elements being oxidized by the.
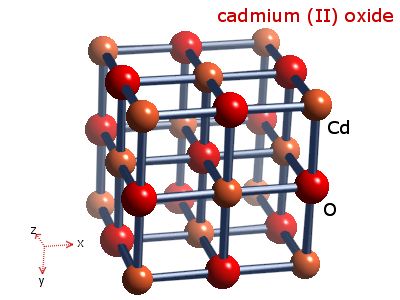 Source: webelements.com
Source: webelements.com
The main shortcoming of the aqueous electrolyte is its small PW approximately 12 V due to water decomposition at 123 V. Solid State ChemiStry and itS appliCation 2014 Anthony R. Solid State ChemiStry and itS appliCation 2014 Anthony R. A naturally occurring oxide sourced from ilmenite rutile and anatase it has a wide range of applications. BCarbon is burnt to provide heat.
 Source: researchgate.net
Source: researchgate.net
Prepared nano zinc oxide by a solid-state pyrolytic method. It is used as a pigment under the names titanium white Pigment White 6 PW6. Solid State ChemiStry and itS appliCation 2014 Anthony R. It has a role as a food colouring. Solid State ChemiStry and itS appliCation 2014 Anthony R.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Residues of calcium oxide are exempted from the requirement of a tolerance when used in accordance with good agricultural practice as inert or occasionally active ingredients in pesticide formulations applied to growing crops or to raw agricultural commodities after harvest. The researchers used this nano zinc oxide as a curing agent in neoprene rubber. Oxide itself is the dianion of oxygen an O 2 molecular ion. It has a role as a food colouring. A naturally occurring oxide sourced from ilmenite rutile and anatase it has a wide range of applications.
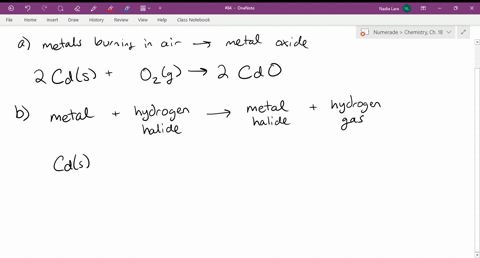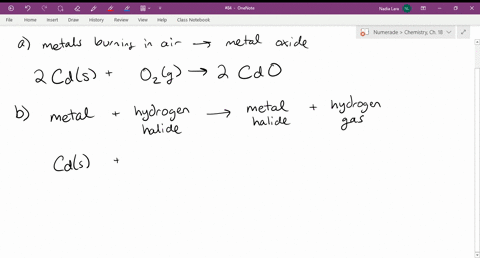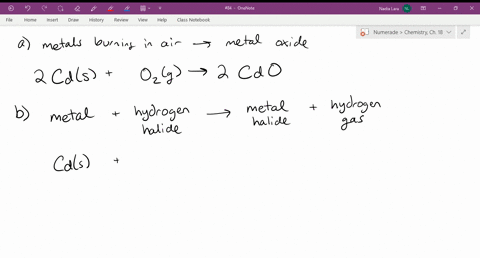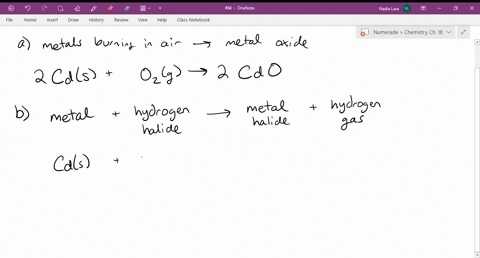 Source: numerade.com
Source: numerade.com
ACalcium oxide reacts with basic impurities. Solid State ChemiStry and itS appliCation 2014 Anthony R. The researchers used this nano zinc oxide as a curing agent in neoprene rubber. Manganese dioxide is the inorganic compound with the formula MnO 2This blackish or brown solid occurs naturally as the mineral pyrolusite which is the main ore of manganese and a component of manganese nodulesThe principal use for MnO 2 is for dry-cell batteries such as the alkaline battery and the zinccarbon battery. A naturally occurring oxide sourced from ilmenite rutile and anatase it has a wide range of applications.
 Source: researchgate.net
Source: researchgate.net
The researchers used this nano zinc oxide as a curing agent in neoprene rubber. Most of the Earths crust consists of solid oxides the result of elements being oxidized by the. Organic electrolytes have smaller electrical conductivity 10 to 60 mScm 1 so lower P d but have higher E d due to wide PW of 2527 V. The main shortcoming of the aqueous electrolyte is its small PW approximately 12 V due to water decomposition at 123 V. Manganese dioxide is the inorganic compound with the formula MnO 2This blackish or brown solid occurs naturally as the mineral pyrolusite which is the main ore of manganese and a component of manganese nodulesThe principal use for MnO 2 is for dry-cell batteries such as the alkaline battery and the zinccarbon battery.
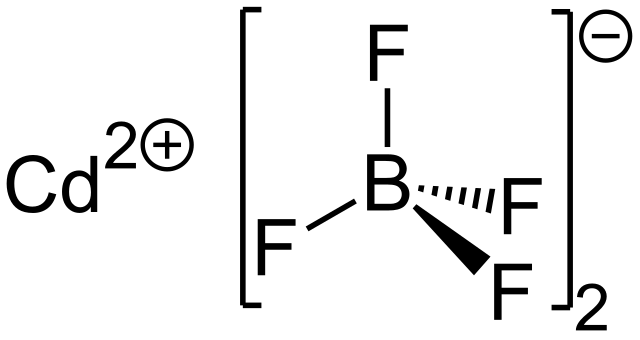 Source: en.wikipedia.org
Source: en.wikipedia.org
Manganese dioxide is the inorganic compound with the formula MnO 2This blackish or brown solid occurs naturally as the mineral pyrolusite which is the main ore of manganese and a component of manganese nodulesThe principal use for MnO 2 is for dry-cell batteries such as the alkaline battery and the zinccarbon battery. Oxide itself is the dianion of oxygen an O 2 molecular ion. 28 Full PDFs related to this paper. Prepared nano zinc oxide by a solid-state pyrolytic method. 29 Which process is used to convert calcium carbonate into calcium oxide.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Full PDF Package Download Full PDF Package. It is used as a pigment under the names titanium white Pigment White 6 PW6. Most of the Earths crust consists of solid oxides the result of elements being oxidized by the. MnO 2 is also used as a pigment and as a precursor to other manganese. Full PDF Package Download Full PDF Package.
 Source: researchgate.net
Source: researchgate.net
A naturally occurring oxide sourced from ilmenite rutile and anatase it has a wide range of applications. ACalcium oxide reacts with basic impurities. The optimum dosage of. 29 Which process is used to convert calcium carbonate into calcium oxide. Sabura et al.
 Source: en.wikipedia.org
Source: en.wikipedia.org
28 Full PDFs related to this paper. An oxide ˈ ɒ k s aɪ d is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. A naturally occurring oxide sourced from ilmenite rutile and anatase it has a wide range of applications. Solid State ChemiStry and itS appliCation 2014 Anthony R. ACalcium oxide reacts with basic impurities.
 Source: researchgate.net
Source: researchgate.net
The main shortcoming of the aqueous electrolyte is its small PW approximately 12 V due to water decomposition at 123 V. 29 Which process is used to convert calcium carbonate into calcium oxide. It has a role as a food colouring. ACalcium oxide reacts with basic impurities. An oxide ˈ ɒ k s aɪ d is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title solid cadmium oxide decomposition formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.