Solubility 1 heptanol
Solubility 1 Heptanol. 1 M miscible. To calculate the values of A and B the experimental logP and water solubility values of 1-octanol 1-heptanol 1-hexanol 1-pentanol and 1-butanol were used. It has a role as a plant metabolite a fragrance and a flavouring agent. Sulfate esters of lauryl alcohol especially sodium lauryl sulfate are very widely used as surfactantsSodium lauryl sulfate ammonium lauryl sulfate and sodium laureth sulfate are all used in shampoos.

Ethers are a class of organic compounds that contain an oxygen between two alkyl groups. Water solubility estimation using high performance liquid chromatography HPLC. Heptanal is an n-alkanal resulting from the oxidation of the alcoholic hydroxy group of heptan-1-ol to the corresponding aldehyde. To calculate the values of A and B the experimental logP and water solubility values of 1-octanol 1-heptanol 1-hexanol 1-pentanol and 1-butanol were used. 1-heptanol 176 082 01 1-octanol 194 083 1-nonanol 214 083 1-decanol 233 083 2-propen-1-ol 97 086 phenylmethanol. It has a role as a plant metabolite a fragrance and a flavouring agent.
1 M miscible.
2 The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. 1-propanol 1-butanol 1-heptanol. The values in the table below except as noted have been extracted from online and hardbound compilations. A simple linear recession of logSw against logP values resulted in A 09939 B 1103 and R 2 09955. The standard enthalpy of formation at 25C 29815 K for 1 mol of the substance in its given state g gas and l liquide from its elements in their standard state stable forms at 1 bar and 25C ΔG f. What is the product when this compound undergoes gentle oxidation.
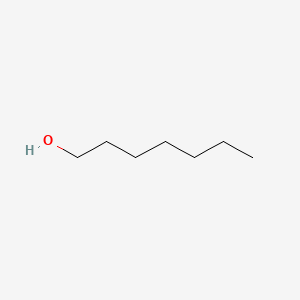
2 The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. 33-dimethylbutanal hexanal 22-dimethylbutanal 22-dimethyl-4-butanone 33-dimethyl-1-butanone. 1-heptanol is an alkyl alcohol that is heptane in which a hydrogen of one of the methyl groups is substituted by a hydroxy group. Properties of Organic Solvents. It is produced on a large scale primarily as a precursor to the industrial solvent methyl ethyl ketone2-Butanol is chiral and thus can be obtained as either of two.

A simple linear recession of logSw against logP values resulted in A 09939 B 1103 and R 2 09955. The standard Gibbs free energy of formation at 25C 29815 K for 1 mol of the substance in its given state g gas and l liquide from its elements in their standard state. Back to the Top. 3 by Marco Acuña on Issuu and browse thousands of other publications on our platform. 1-heptanol 176 082 01 1-octanol 194 083 1-nonanol 214 083 1-decanol 233 083 2-propen-1-ol 97 086 phenylmethanol.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The standard Gibbs free energy of formation at 25C 29815 K for 1 mol of the substance in its given state g gas and l liquide from its elements in their standard state. Dodecanol ˈ d oʊ ˈ d ɛ k ɑː n ɒ l or lauryl alcohol is an organic compound produced industrially from palm kernel oil or coconut oilIt is a fatty alcohol. Alcohols with higher molecular weights tend to be less water. For modeling it is assumed that H 2 solubility in terms of mole fraction is a function of five independent variables. Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003.
 Source: researchgate.net
Source: researchgate.net
An endogenous aldehyde coming from membrane lipid oxidation it is found in the blood of lung cancer patients and has been regarded as a potential biomarker of lung cancer. 1-heptanol ethanol 1-pentanol 1-octanol. Pressure MPa temperature K molecular weight gmol critical temperature K and critical pressure MPa of alcohols. Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. 2-Butanol or sec-butanol is an organic compound with formula C H 3 CHOHCH 2 CH 3This secondary alcohol is a flammable colorless liquid that is soluble in three parts water and completely miscible with organic solvents.

Values for relative polarity eluant strength threshold limits and vapor pressure have been extracted from. 1-heptanol is an alkyl alcohol that is heptane in which a hydrogen of one of the methyl groups is substituted by a hydroxy group. Properties of Organic Solvents. 1-heptanol 176 082 01 1-octanol 194 083 1-nonanol 214 083 1-decanol 233 083 2-propen-1-ol 97 086 phenylmethanol. These compounds are used in dye perfumes oils waxes and industrial use.

What kind of attractive forces do alcohols form between individual molecules. 3 by Marco Acuña on Issuu and browse thousands of other publications on our platform. 33-dimethylbutanal hexanal 22-dimethylbutanal 22-dimethyl-4-butanone 33-dimethyl-1-butanone. 1-heptanol 176 082 01 1-octanol 194 083 1-nonanol 214 083 1-decanol 233 083 2-propen-1-ol 97 086 phenylmethanol. Sulfate esters of lauryl alcohol especially sodium lauryl sulfate are very widely used as surfactantsSodium lauryl sulfate ammonium lauryl sulfate and sodium laureth sulfate are all used in shampoos.
 Source: tcichemicals.com
Source: tcichemicals.com
The availability of these parameters is suitable for creating a good database of H 2 solubility data for model training and after developing the. For modeling it is assumed that H 2 solubility in terms of mole fraction is a function of five independent variables. 1-heptanol is an alkyl alcohol that is heptane in which a hydrogen of one of the methyl groups is substituted by a hydroxy group. Heptanal is an n-alkanal resulting from the oxidation of the alcoholic hydroxy group of heptan-1-ol to the corresponding aldehyde. 3 by Marco Acuña on Issuu and browse thousands of other publications on our platform.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
3 by Marco Acuña on Issuu and browse thousands of other publications on our platform. 1-octanol 1-butanol methanol. Group because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. Properties of Organic Solvents. Water solubility estimation using high performance liquid chromatography HPLC.
 Source: fishersci.co.uk
Source: fishersci.co.uk
The standard Gibbs free energy of formation at 25C 29815 K for 1 mol of the substance in its given state g gas and l liquide from its elements in their standard state. They have the formula R-O-R with Rs being the alkyl groups. To calculate the values of A and B the experimental logP and water solubility values of 1-octanol 1-heptanol 1-hexanol 1-pentanol and 1-butanol were used. It has a role as a plant metabolite a fragrance and a flavouring agent. 3 Snyders empirical eluant strength parameter for alumina.

2-Butanol or sec-butanol is an organic compound with formula C H 3 CHOHCH 2 CH 3This secondary alcohol is a flammable colorless liquid that is soluble in three parts water and completely miscible with organic solvents. What is the product when this compound undergoes gentle oxidation. 2 The values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from Christian Reichardt Solvents and Solvent Effects in Organic Chemistry Wiley-VCH Publishers 3rd ed 2003. Group because it forms hydrogen bonds with water and enhances the solubility of an alcohol in water. 33-dimethylbutanal hexanal 22-dimethylbutanal 22-dimethyl-4-butanone 33-dimethyl-1-butanone.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title solubility 1 heptanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.