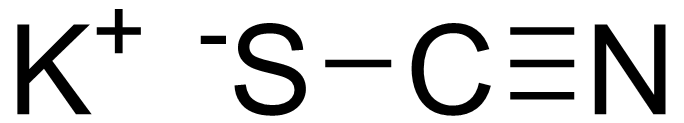Solubility calcium nitrate
Solubility Calcium Nitrate. Solubility is the ability of a solid liquid or gaseous chemical substance referred to as the solute to dissolve in solvent usually a. Aqueous silver nitrate AgNO 3 is added to a solution containing potassium chloride. Ksp solubility product constants of many popular salts at SolubilityOFthings. An equilibrium constant is the ratio of the concentration of the products of a reaction divided by the concentration of the reactants once the reaction has reached equilibrium.
 Wo2006031139a1 Method Of Calcium Nitrate Production Google Patents From patents.google.com
Wo2006031139a1 Method Of Calcium Nitrate Production Google Patents From patents.google.com
Several million kilograms are produced annually for this purpose. The solubility product constant K sp is the equilibrium constant for a solid that dissolves in an aqueous solutionAll of the rules for determining equilibrium constants continue to apply. The preparation of BaNO32 by double conversion of barium chloride and sodium nitrate or calcium nitrate is described in the Eastern European patent literature. The second major application of nitrates is as. The relative ability of a solute to dissolve into a solvent. Especially in bone formation and maintenance.
Nitrates are also used in fertilizer because of their biodegradable properties and their high solubility.
The solubility product constant K sp is the equilibrium constant for a solid that dissolves in an aqueous solutionAll of the rules for determining equilibrium constants continue to apply. Solubility in water as weight. Several million kilograms are produced annually for this purpose. The primary nitrate fertilizers include calcium salts potassium ammonium and sodium. Nitrate in the real samples will interfere with the detection of nitrite. John Wiley.
 Source: patents.google.com
Source: patents.google.com
Aluminium nitrate is a strong oxidizing agent. Worked Example 1 using the StoPGoPS approach to problem solving. Decreasing solubility with increasing temperature. Essentially all alkali metal Li Na K Rb Cs and ammonium NH 4. Especially in bone formation and maintenance.

Therefore when there is a Calcium deficiency when the correct amount of Calcium is not taken in diet Calcium is taken. Ullmanns Encyclopedia of Industrial Chemistry. All nitrate NO 3 nitrite NO 2 chlorate ClO 3 and perchlorate ClO 4 salts are soluble. The solubility of calcium hydroxide at 25C is 012 g100 mL water. The solubility product constant K sp is the equilibrium constant for a solid that dissolves in an aqueous solutionAll of the rules for determining equilibrium constants continue to apply.
 Source: chemix-chemistry-software.com
Source: chemix-chemistry-software.com
Worked Example 1 using the StoPGoPS approach to problem solving. Several million kilograms are produced annually for this purpose. An equilibrium constant is the ratio of the concentration of the products of a reaction divided by the concentration of the reactants once the reaction has reached equilibrium. Nitrate salts are found naturally on earth as large deposits. Solubility in water as weight.
 Source: twitter.com
Source: twitter.com
What Is The Formal Charge Of NO3. All nitrate NO 3 nitrite NO 2 chlorate ClO 3 and perchlorate ClO 4 salts are soluble. The Lewis representation of NO3 is aligned in. The second major application of nitrates is as. Ammonium nitrate dissolving in solution is an endothermic reaction.
 Source: sciencedirect.com
Source: sciencedirect.com
What Is The Formal Charge Of NO3. Decreasing solubility with increasing temperature. The relative ability of a solute to dissolve into a solvent. Ksp solubility product constants of many popular salts at SolubilityOFthings. Nitrates are mainly produced for use as fertilizers in agriculture because of their high solubility and biodegradability.
 Source: sciencedirect.com
Source: sciencedirect.com
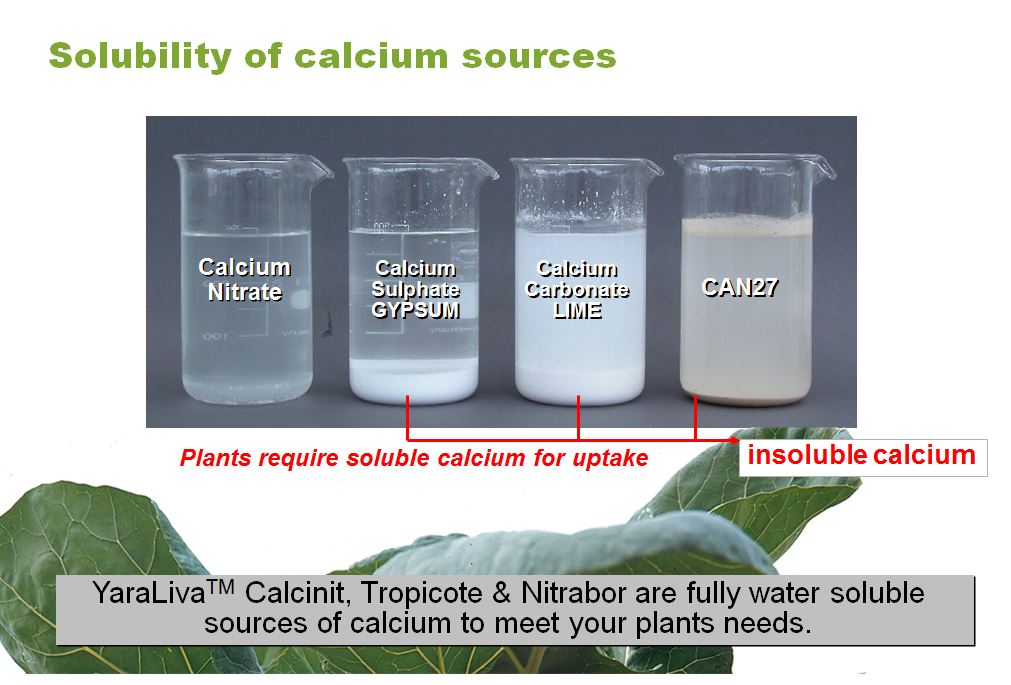
Calcium carbonate calcium citrate calcium nitrate calcium sulfide. Nitrates are also used in fertilizer because of their biodegradable properties and their high solubility. The Lewis representation of NO3 is aligned in. What Is The Formal Charge Of NO3. The solubility product constant K sp is the equilibrium constant for a solid that dissolves in an aqueous solutionAll of the rules for determining equilibrium constants continue to apply.

The compound usually a liquid that dissolves the solute. Ammonium nitrate dissolving in solution is an endothermic reaction. It is a white powder by nature and has poor solubility in water. Nitrates are mainly produced for use as fertilizers in agriculture because of their high solubility and biodegradability. Flowers pretreated with 0125 M calcium nitrate appeared translucent or water-soaked after 5 days at -4 C.
 Source: sciencedirect.com
Source: sciencedirect.com
Calcium carbonate calcium citrate calcium nitrate calcium sulfide. 127 developed a new BODIPY reagent 135-tetramethyl-8-4-aminophenyl-44-dilfluoro-4-bora-3a4a-diaza- s - indacence TMABODIPY to determine trace nitrite in terms of the reaction of nitrite with TMABODIPY first in acidic solution and then in alkaline solution to for diazonate a stable and extremely. Kresse R et al. Worked Example 1 using the StoPGoPS approach to problem solving. John Wiley.
 Source: sciencedirect.com
Source: sciencedirect.com
Silver nitrite and potassium perchlorate are considered slightly soluble. What Is The Formal Charge Of NO3. Nitrates are mainly produced for use as fertilizers in agriculture because of their high solubility and biodegradability. Calcium carbonate calcium citrate calcium nitrate calcium sulfide. Silver nitrite and potassium perchlorate are considered slightly soluble.
 Source: calcium.atomistry.com
Source: calcium.atomistry.com
John Wiley. Essentially all alkali metal Li Na K Rb Cs and ammonium NH 4. Decreasing solubility with increasing temperature. John Wiley. Kresse R et al.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title solubility calcium nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.