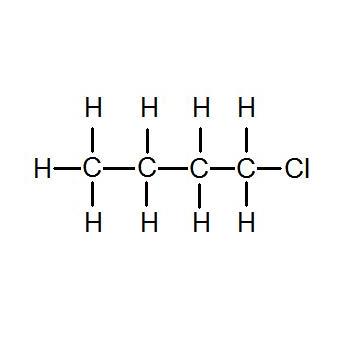
Solubility of aldehyde in ethanol
Solubility Of Aldehyde In Ethanol. J Pharm Sci 70. Cinnamaldehyde is an organic compound with the formula C 6 H 5 CHCHCHO. Silver nitrate is an inorganic compound with chemical formula AgNO 3This salt is a versatile precursor to many other silver compounds such as those used in photographyIt is far less sensitive to light than the halidesIt was once called lunar caustic because silver was called luna by the ancient alchemists who associated silver with the moon. In solid silver nitrate the silver ions are.
 Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry From wou.edu
Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry From wou.edu
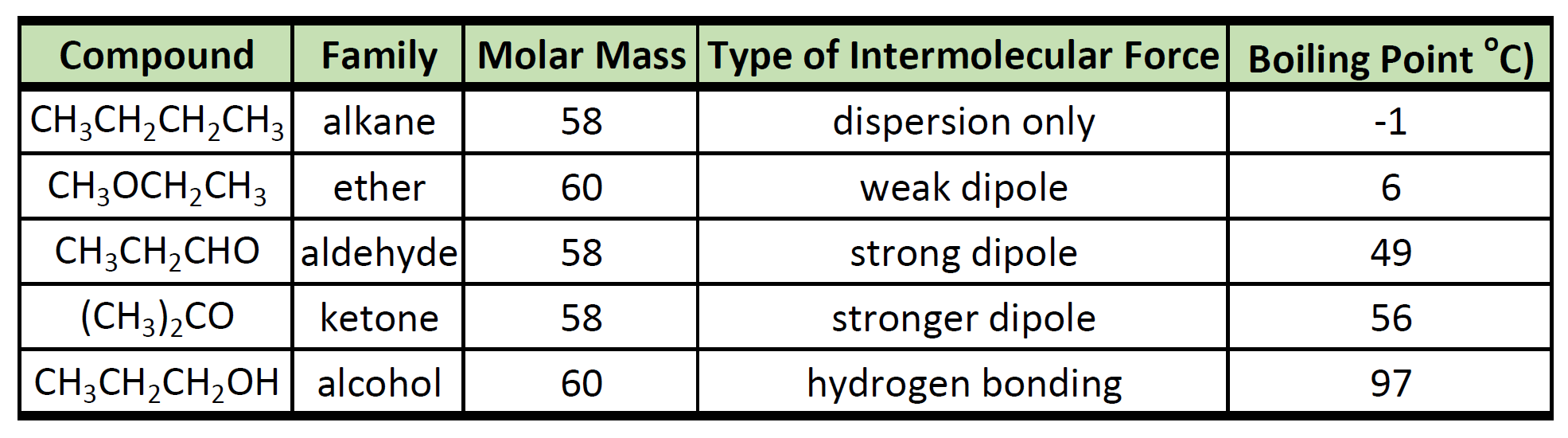
A ketone carbonyl function may be located anywhere within a chain or ring and its position is given by a locator number. There is no hydrogen bonded to a highly electronegative atom. Silver nitrate is an inorganic compound with chemical formula AgNO 3This salt is a versatile precursor to many other silver compounds such as those used in photographyIt is far less sensitive to light than the halidesIt was once called lunar caustic because silver was called luna by the ancient alchemists who associated silver with the moon. Chain numbering normally starts from the end nearest the carbonyl group. Since an aldehyde carbonyl group must always lie at the end of a carbon chain it is by default position 1 and therefore defines the numbering direction. They have a lower bp.
In cyclic ketones the carbonyl group is.
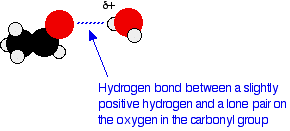
The Chemical Rubber Co 1975 p. 502-7 1981 Hazardous Substances Data Bank. Although polar they do not form hydrogen bonds reason. There is no hydrogen bonded to a highly electronegative atom. J Pharm Sci 70. Since an aldehyde carbonyl group must always lie at the end of a carbon chain it is by default position 1 and therefore defines the numbering direction.
 Source: slideplayer.com
Source: slideplayer.com
Ethanol use is associated with skin irritation or contact dermatitis especially in humans with an aldehyde dehydrogenase ALDH deficiency. They have a lower bp. Esters can be used as solvents for polar organic substances. Silver nitrate is an inorganic compound with chemical formula AgNO 3This salt is a versatile precursor to many other silver compounds such as those used in photographyIt is far less sensitive to light than the halidesIt was once called lunar caustic because silver was called luna by the ancient alchemists who associated silver with the moon. There is no hydrogen bonded to a highly electronegative atom.
 Source: wou.edu
Source: wou.edu
We will guide you on how to place your essay help proofreading and editing your draft fixing the grammar spelling or formatting of your paper easily and cheaply. J Pharm Sci 70. Ethyl ethanoate is used as a solvent in glues and printing inks. They have a lower bp. Occurring naturally as predominantly the trans isomer it gives cinnamon its flavor and odor.
 Source: youtube.com
Source: youtube.com
In cyclic ketones the carbonyl group is. Filter off the crystals wash with a little cold water and recrystallise from methanol or ethanol. Shake the mixture for a few minutes and then cool in ice-water. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. Get 247 customer support help when you place a homework help service order with us.
 Source: slideplayer.com
Source: slideplayer.com
Ethanol use is associated with skin irritation or contact dermatitis especially in humans with an aldehyde dehydrogenase ALDH deficiency. Edited translated and revised by TE. They have a lower bp. Ethyl ethanoate is used as a solvent in glues and printing inks. The Chemical Rubber Co 1975 p.
 Source: sciencedirect.com
Source: sciencedirect.com
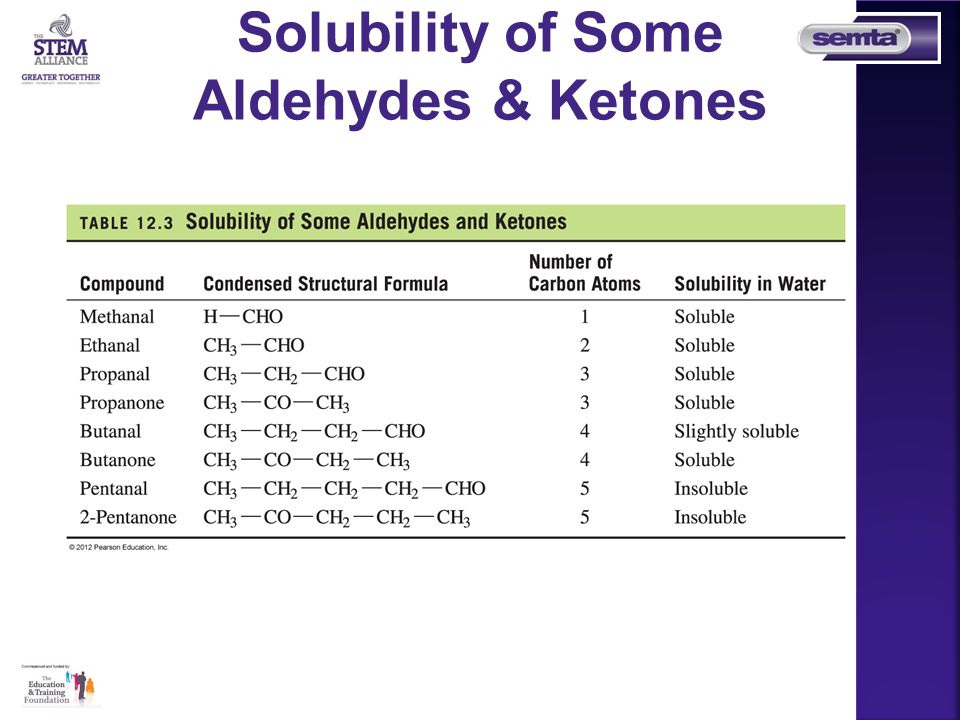
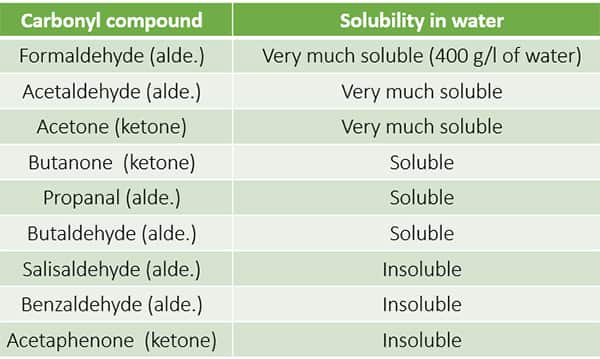
Solubility in Water The smaller carboxylic up to C4. Solubility in Water The smaller carboxylic up to C4. Ethanol use is associated with skin irritation or contact dermatitis especially in humans with an aldehyde dehydrogenase ALDH deficiency. The Chemical Rubber Co 1975 p. Occurring naturally as predominantly the trans isomer it gives cinnamon its flavor and odor.
 Source: slideplayer.com
Source: slideplayer.com
Edited translated and revised by TE. A ketone carbonyl function may be located anywhere within a chain or ring and its position is given by a locator number. Although polar they do not form hydrogen bonds reason. Cinnamaldehyde is an organic compound with the formula C 6 H 5 CHCHCHO. In the form of hand disinfectants relatively low but measurable blood concentrations of ethanol and its metabolite acetaldehyde may occur which are however below acute toxic levels.
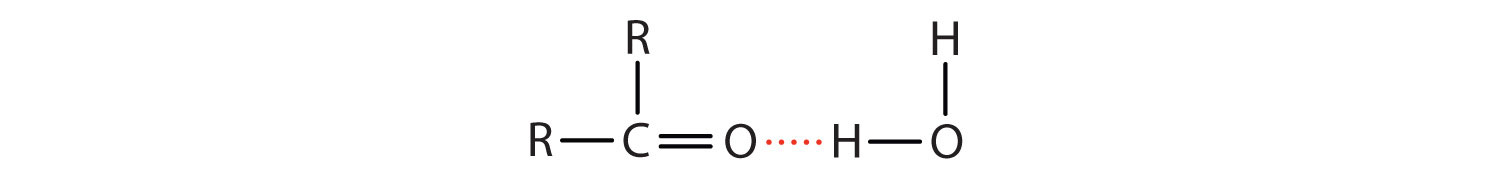 Source: chem.libretexts.org
Source: chem.libretexts.org
It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. Ethanol use is associated with skin irritation or contact dermatitis especially in humans with an aldehyde dehydrogenase ALDH deficiency. After regular application of ethanol on the skin eg. There is no hydrogen bonded to a highly electronegative atom. The Chemical Rubber Co 1975 p.
 Source: chemistryscl.com
Source: chemistryscl.com
Silver nitrate is an inorganic compound with chemical formula AgNO 3This salt is a versatile precursor to many other silver compounds such as those used in photographyIt is far less sensitive to light than the halidesIt was once called lunar caustic because silver was called luna by the ancient alchemists who associated silver with the moon. J Pharm Sci 70. Shake the mixture for a few minutes and then cool in ice-water. Although polar they do not form hydrogen bonds reason. In the form of hand disinfectants relatively low but measurable blood concentrations of ethanol and its metabolite acetaldehyde may occur which are however below acute toxic levels.
 Source: chemguide.co.uk
Source: chemguide.co.uk
In the form of hand disinfectants relatively low but measurable blood concentrations of ethanol and its metabolite acetaldehyde may occur which are however below acute toxic levels. Ethanol volatile turns into gas easily and not react with water. In the form of hand disinfectants relatively low but measurable blood concentrations of ethanol and its metabolite acetaldehyde may occur which are however below acute toxic levels. Solubility in Water The smaller carboxylic up to C4. Get 247 customer support help when you place a homework help service order with us.
 Source: chem.libretexts.org
Source: chem.libretexts.org
This pale yellow viscous liquid occurs in the bark of cinnamon trees and other species of the genus CinnamomumThe essential oil of cinnamon bark is. In the form of hand disinfectants relatively low but measurable blood concentrations of ethanol and its metabolite acetaldehyde may occur which are however below acute toxic levels. Filter off the crystals wash with a little cold water and recrystallise from methanol or ethanol. Shake the mixture for a few minutes and then cool in ice-water. After regular application of ethanol on the skin eg.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title solubility of aldehyde in ethanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.