Solubility of naphthalene
Solubility Of Naphthalene. 2 C 6 Cl 2 CN 2 O 2 C 10 H 12 2 C 6 Cl 2 CN 2 OH 2 C 10 H 8. C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. For those that are molecular we can predict whether. The two phases can then be physically separated.
 A Estimate Of Dependence Of Water Solubility In Mg L Of Naphthalene Download Scientific Diagram From researchgate.net
A Estimate Of Dependence Of Water Solubility In Mg L Of Naphthalene Download Scientific Diagram From researchgate.net
C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. The reagent removes pairs of H atoms from organic molecules. The two phases can then be physically separated. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. 2 C 6 Cl 2 CN 2 O 2 C 10 H 12 2 C 6 Cl 2 CN 2 OH 2 C 10 H 8. The resulting hydroquinone is poorly soluble in typical reaction solvents dioxane benzene alkanes which.
C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1.
If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular. The reagent removes pairs of H atoms from organic molecules.

Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. 2 C 6 Cl 2 CN 2 O 2 C 10 H 12 2 C 6 Cl 2 CN 2 OH 2 C 10 H 8. The two phases can then be physically separated.
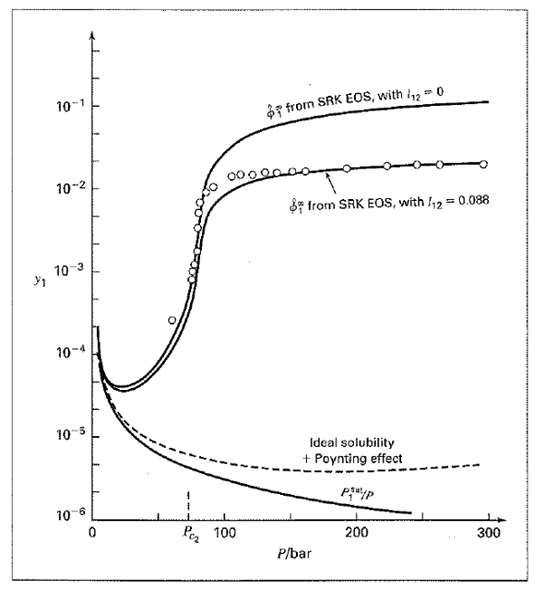 Source: chegg.com
Source: chegg.com
C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. 2 C 6 Cl 2 CN 2 O 2 C 10 H 12 2 C 6 Cl 2 CN 2 OH 2 C 10 H 8. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. The resulting hydroquinone is poorly soluble in typical reaction solvents dioxane benzene alkanes which.
 Source: researchgate.net
Source: researchgate.net
For those that are molecular we can predict whether. The resulting hydroquinone is poorly soluble in typical reaction solvents dioxane benzene alkanes which. For those that are molecular we can predict whether. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene.
 Source: researchgate.net
Source: researchgate.net
C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. The reagent removes pairs of H atoms from organic molecules. The resulting hydroquinone is poorly soluble in typical reaction solvents dioxane benzene alkanes which. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene.
 Source: researchgate.net
Source: researchgate.net
Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular. If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. Sample Exercise 131 Predicting Solubility Patterns Solution Analyze We are given two solvents one that is nonpolar CCl 4 and the other that is polar H 2 O and asked to determine which will be the better solvent for each solute listed.
 Source: researchgate.net
Source: researchgate.net
C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. Sample Exercise 131 Predicting Solubility Patterns Solution Analyze We are given two solvents one that is nonpolar CCl 4 and the other that is polar H 2 O and asked to determine which will be the better solvent for each solute listed. The two phases can then be physically separated. C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular.
 Source: researchgate.net
Source: researchgate.net
- hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular. For those that are molecular we can predict whether. If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby.
 Source: chegg.com
Source: chegg.com
Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular. The resulting hydroquinone is poorly soluble in typical reaction solvents dioxane benzene alkanes which. If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby. Plan By examining the formulas of the solutes we can predict whether they are ionic or molecular.

2 C 6 Cl 2 CN 2 O 2 C 10 H 12 2 C 6 Cl 2 CN 2 OH 2 C 10 H 8. The two phases can then be physically separated. 2 C 6 Cl 2 CN 2 O 2 C 10 H 12 2 C 6 Cl 2 CN 2 OH 2 C 10 H 8. C-17 Solubility of Sulfur Compounds in Water as a Function of Boiling Point for Mercaptans and Aromatics C-18 Solubility of Naphthenes in Water C-19 Solubility of Nitrogen Compounds in Water C-20 Henrys Law Constant for Nitrogen Compounds in Water C-21 Coefficient of Thermal Expansion of Liquids C-22 Adsorption Capacity of Activated Carbon FURTHER READING 1. - hig h ether solubility - low water solubility-h igp olrtyHbnd - lowe trsubiy-h igwa tersouby.
 Source: rkt.chem.ox.ac.uk
Source: rkt.chem.ox.ac.uk
If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. If the mixture of naphthalene and glucose is dissolved in tert-butyl methyl ether and mixed well with water the glucose will mostly dissolve in the lower water phase phase layer and the naphthalene will mostly dissolve in the upper ether phase. The reagent removes pairs of H atoms from organic molecules. The resulting hydroquinone is poorly soluble in typical reaction solvents dioxane benzene alkanes which. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title solubility of naphthalene by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.