Solubility of organic and inorganic compounds
Solubility Of Organic And Inorganic Compounds. The carbon atom of this group has two remaining bonds that may be occupied by hydrogen or alkyl or aryl substituents. Do not weigh the solid. Aerosol particles frequently contain mixtures of water organic compounds and inorganic ions so we have extended the thermodynamics-based group-contribution model AIOMFAC-VISC to predict viscosity for aqueous electrolyte solutions and aqueous organic-inorganic mixtures. Procedure for Determining Solubility of Organic Compounds The amounts of material to use for a solubility test are somewhat flexible.
 Carbon And Organic Compounds Organic Vs Inorganic Organic Compounds Contain Carbon That Is Covalently Bonded To Non Metals Ppt Download From slideplayer.com
Carbon And Organic Compounds Organic Vs Inorganic Organic Compounds Contain Carbon That Is Covalently Bonded To Non Metals Ppt Download From slideplayer.com
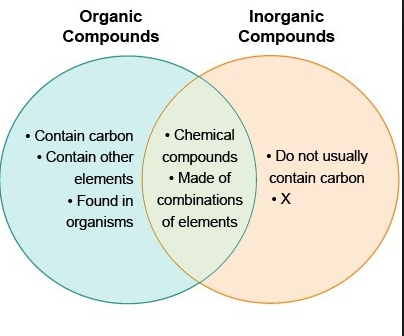
Do not weigh the solid. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group CO. Inorganic compound is a chemical compound which is the opposite of an organic compound. The carbon atom of this group has two remaining bonds that may be occupied by hydrogen or alkyl or aryl substituents. Organic compounds are called those containing mainly carbon and hydrogen atoms in correlation and composition with other elements. Difference Between Organic And Inorganic Compounds - Organic compound is a chemical compound of living things which contains Carbon and carbon atoms because of their relations with organisms.
Use 2-3 drops of a liquid or approximately 10 mg of a solid.
Simply use enough to cover the tip of a small spatula. Do not weigh the solid. For metal-containing compounds that are reactive toward air Schlenk line and glove box techniques are followed. Inorganic compound is a chemical compound which is the opposite of an organic compound. The IUPAC system of nomenclature assigns a. This type of compounds have covalent bonds between nonmetallic atoms of few elements from two to five and they are of great complexity existing around 10 million compounds of this type.
 Source: pediaa.com
Source: pediaa.com
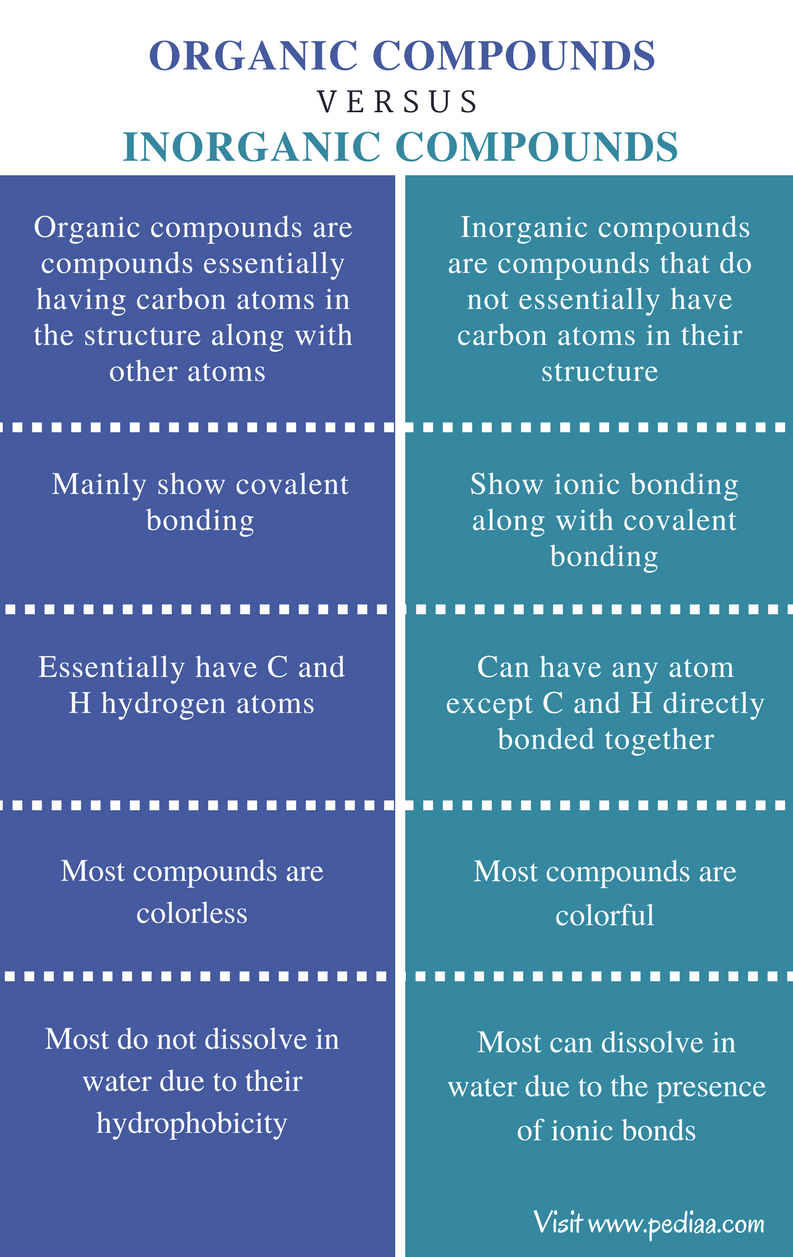
Organic compounds are called those containing mainly carbon and hydrogen atoms in correlation and composition with other elements. Soluble inorganic compounds are prepared using methods of organic synthesis. The technique of recrystallization used for purification of solids depends on a solutes different solubilities in hot and cold solventA few exceptions exist such as certain cyclodextrins. Simply use enough to cover the tip of a small spatula. For metal-containing compounds that are reactive toward air Schlenk line and glove box techniques are followed.
 Source: slideplayer.com
Source: slideplayer.com
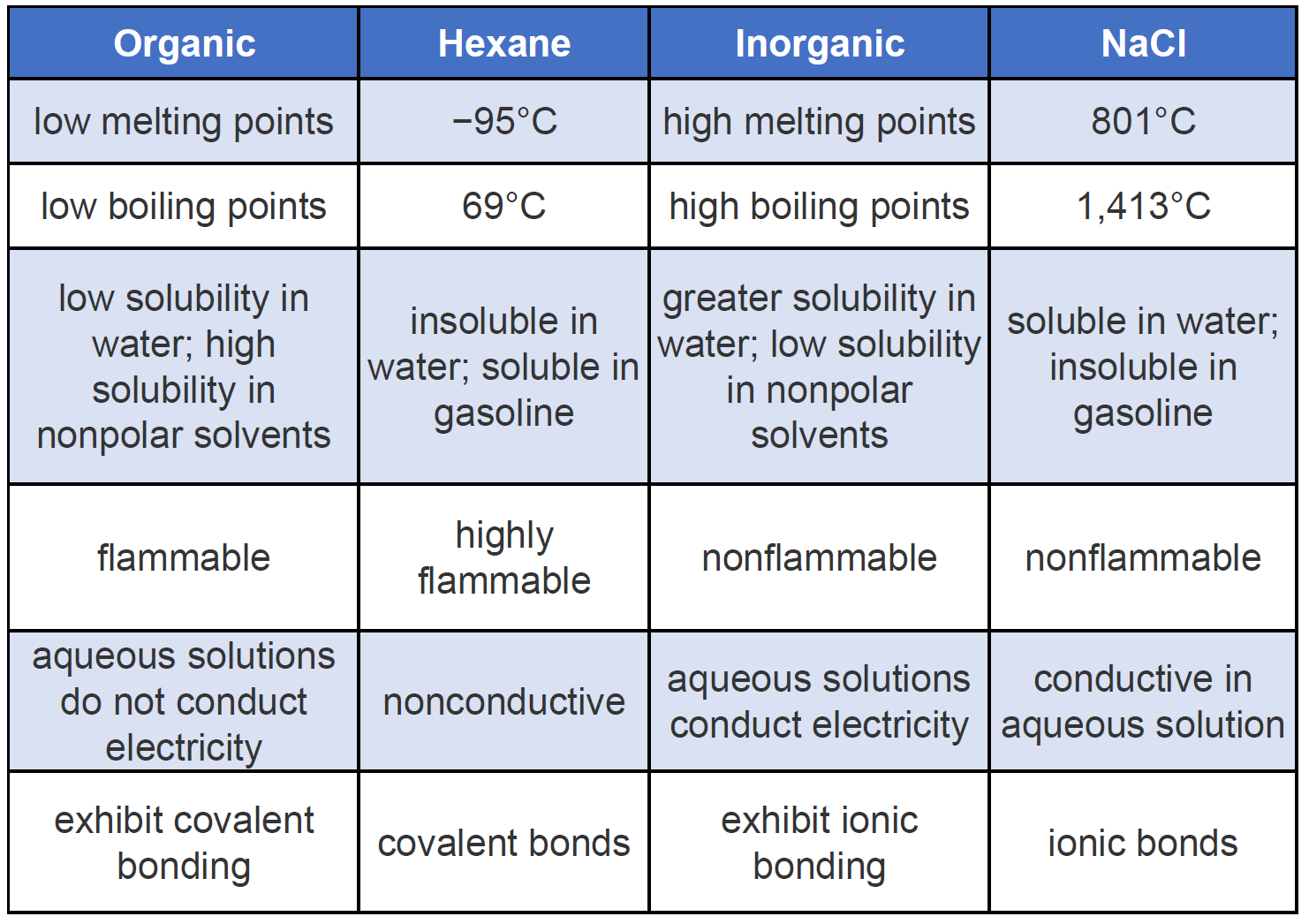
Difference Between Organic And Inorganic Compounds - Organic compound is a chemical compound of living things which contains Carbon and carbon atoms because of their relations with organisms. Comparison of properties between ionic inorganic and covalent organic compounds. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group CO. The solubility of organic compounds nearly always increases with temperature. For condensed phases solids and liquids the pressure dependence of solubility is typically weak and usually.
 Source: schoolworkhelper.net
Source: schoolworkhelper.net
Inorganic synthetic methods can be classified roughly according to the volatility or solubility of the component reactants. For condensed phases solids and liquids the pressure dependence of solubility is typically weak and usually. This type of compounds have covalent bonds between nonmetallic atoms of few elements from two to five and they are of great complexity existing around 10 million compounds of this type. Do not weigh the solid. It can be considered as a compound which does not contain carbon to hydrogen bond which calls C-H bond.
 Source: slideplayer.com
Source: slideplayer.com
Organic compounds are called those containing mainly carbon and hydrogen atoms in correlation and composition with other elements. Organic compounds are called those containing mainly carbon and hydrogen atoms in correlation and composition with other elements. For metal-containing compounds that are reactive toward air Schlenk line and glove box techniques are followed. For condensed phases solids and liquids the pressure dependence of solubility is typically weak and usually. For aqueous electrolyte solutions our new semi-empirical approach uses a physical expression based on Eyrings.
 Source: pinterest.com
Source: pinterest.com
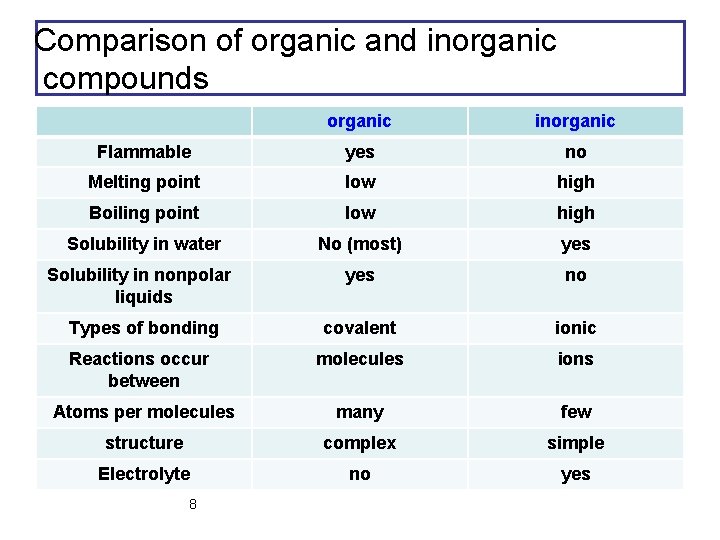
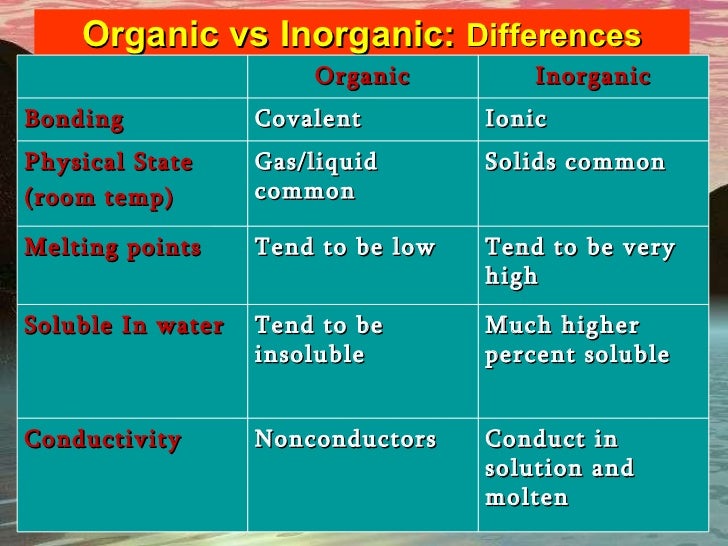
If neither is hydrogen the compound is a ketone. Property Inorganic Organic melting point High Low boiling point High Low solubility in H2O generally soluble generally insoluble flammability nonflammable flammable rate of chemical reactivity often fast often fast ability to conduct in solution conductors in solution nonconductors in solution. The solubility of organic compounds nearly always increases with temperature. Use 2-3 drops of a liquid or approximately 10 mg of a solid. Unless the solid is already a fine powder crush a small amount of the solid on a watch glass with the back of a spatula.
 Source: wou.edu
Source: wou.edu
This type of compounds have covalent bonds between nonmetallic atoms of few elements from two to five and they are of great complexity existing around 10 million compounds of this type. If neither is hydrogen the compound is a ketone. The technique of recrystallization used for purification of solids depends on a solutes different solubilities in hot and cold solventA few exceptions exist such as certain cyclodextrins. The solubility of organic compounds nearly always increases with temperature. For metal-containing compounds that are reactive toward air Schlenk line and glove box techniques are followed.
 Source: slidetodoc.com
Source: slidetodoc.com
The IUPAC system of nomenclature assigns a. Property Inorganic Organic melting point High Low boiling point High Low solubility in H2O generally soluble generally insoluble flammability nonflammable flammable rate of chemical reactivity often fast often fast ability to conduct in solution conductors in solution nonconductors in solution. Simply use enough to cover the tip of a small spatula. Organic compounds are called those containing mainly carbon and hydrogen atoms in correlation and composition with other elements. Volatile compounds and gases are.
 Source: slideplayer.com
Source: slideplayer.com
Organic compounds are called those containing mainly carbon and hydrogen atoms in correlation and composition with other elements. Volatile compounds and gases are. Aldehydes and ketones are organic compounds which incorporate a carbonyl functional group CO. Difference Between Organic And Inorganic Compounds - Organic compound is a chemical compound of living things which contains Carbon and carbon atoms because of their relations with organisms. For metal-containing compounds that are reactive toward air Schlenk line and glove box techniques are followed.
 Source: vivadifferences.com
Source: vivadifferences.com
Procedure for Determining Solubility of Organic Compounds The amounts of material to use for a solubility test are somewhat flexible. This type of compounds have covalent bonds between nonmetallic atoms of few elements from two to five and they are of great complexity existing around 10 million compounds of this type. Procedure for Determining Solubility of Organic Compounds The amounts of material to use for a solubility test are somewhat flexible. The solubility of organic compounds nearly always increases with temperature. For condensed phases solids and liquids the pressure dependence of solubility is typically weak and usually.
 Source: sites.google.com
Source: sites.google.com
Difference Between Organic And Inorganic Compounds - Organic compound is a chemical compound of living things which contains Carbon and carbon atoms because of their relations with organisms. Organic compounds are called those containing mainly carbon and hydrogen atoms in correlation and composition with other elements. Volatile compounds and gases are. Procedure for Determining Solubility of Organic Compounds The amounts of material to use for a solubility test are somewhat flexible. Simply use enough to cover the tip of a small spatula.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title solubility of organic and inorganic compounds by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.