Solution of lead nitrate
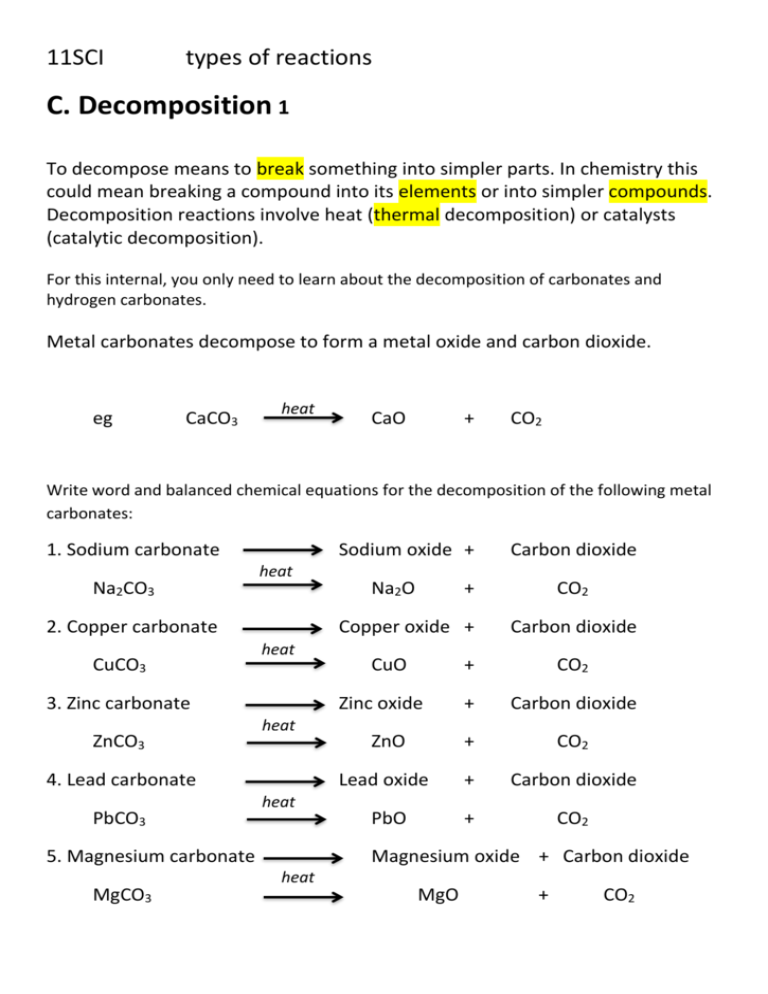
Solution Of Lead Nitrate. A Nitrate Test is a chemical test used to determine the presence of nitrate ion in solution. Our purpose is to solve the toughest problems in life science by collaborating. Apply a cotton applicator dipped in solutionointment on the affected area 2-3 times per week for 2-3 weeks. The pre-mixed materials come fairly close to providing the desired concentrations of micronutrients although some are.
 Lead Nitrate And Sodium Iodide Reaction Youtube From youtube.com
Lead Nitrate And Sodium Iodide Reaction Youtube From youtube.com
We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses. No media kitchen no autoclave no bottles. A few drops of silver nitrate solution are added to the solution and a pale yellow precipitate forms. Using the initial concentrations calculate the reaction quotient Q and compare to the value of the equilibrium constant K sp. Halides precipitate with silver and sulfate precipitate with barium. Chlorine water and carbon tetrachloride were added which resulted in a purple solution.
In contrast many common ions give insoluble salts eg.
Using the initial concentrations calculate the reaction quotient Q and compare to the value of the equilibrium constant K sp. Our purpose is to solve the toughest problems in life science by collaborating. The pre-mixed materials come fairly close to providing the desired concentrations of micronutrients although some are. When Trust and Teamwork Meet. The hydroxide ion forms salts some of which dissociate in aqueous solution. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions as almost all nitrates are soluble in water.
 Source: flinnsci.com
Source: flinnsci.com
The pre-mixed materials come fairly close to providing the desired concentrations of micronutrients although some are. Ammonium nitrate N2H4O3 is one such compound and has an NPK rating of 34-0-0. When Trust and Teamwork Meet. Chlorine water and carbon tetrachloride were added which resulted in a purple solution. A New Way for Making your Enrichment Broth.
 Source: shutterstock.com
Source: shutterstock.com
Our purpose is to solve the toughest problems in life science by collaborating. Ninety percent of synthetic urea produced now is for fertilizers. Each pound of calcium chloride 36 Ca in 30 gallons of stock solution results in a 14 ppm increase in Ca in the final nutrient solution delivered to the plants. Q 00000938 M Pb 2000050 M CrO 4 2- 469 x 10-8 Q is greater than K sp so a precipitate of leadII chromate will form. No media kitchen no autoclave no bottles.
 Source: carolina.com
Source: carolina.com
A Nitrate Test is a chemical test used to determine the presence of nitrate ion in solution. Ammonium nitrate N2H4O3 is one such compound and has an NPK rating of 34-0-0. The hydroxide ion appears to rotate freely in crystals of. Write the balanced equation for the reaction that took place between the salt and silver nitrate. Urea on the other hand has an NPK grade of 46-0-0 making it more economical to transport.
 Source: flinnsci.com
Source: flinnsci.com
White lead is a basic lead carbonate PbCO 3 2 PbOH 2 which has been used as a white pigment because of its opaque quality though its use is now restricted because it can be a source for lead poisoning. In contrast many common ions give insoluble salts eg. A few drops of silver nitrate solution are added to the solution and a pale yellow precipitate forms. Testing for the presence of nitrate via wet chemistry is generally difficult compared with testing for other anions as almost all nitrates are soluble in water. We regularly produce chemical solutions to specifications designed by government and regulatory bodies commercial and trade associations and the specific needs of individual users and businesses.
 Source: socratic.org
Source: socratic.org
A New Way for Making your Enrichment Broth. Our product line consists of chemical solutions prepared to exact quality standards and certified for use in laboratories and production processes. Similar calculation for the leadII nitrate yields. An option to increase the Ca in the solution is to supplement the calcium nitrate stock with calcium chloride. Ammonium nitrate N2H4O3 is one such compound and has an NPK rating of 34-0-0.
 Source: youtube.com
Source: youtube.com
In contrast many common ions give insoluble salts eg. Which one of the following salts was dissolved in the original solution. Chlorine water and carbon tetrachloride were added which resulted in a purple solution. A few drops of silver nitrate solution are added to the solution and a pale yellow precipitate forms. Using the initial concentrations calculate the reaction quotient Q and compare to the value of the equilibrium constant K sp.
 Source: youtube.com
Source: youtube.com
No media kitchen no autoclave no bottles. Which one of the following salts was dissolved in the original solution. No media kitchen no autoclave no bottles. The pre-mixed materials come fairly close to providing the desired concentrations of micronutrients although some are. Ammonium nitrate N2H4O3 is one such compound and has an NPK rating of 34-0-0.

Ninety percent of synthetic urea produced now is for fertilizers. The hydroxide ion forms salts some of which dissociate in aqueous solution. A few drops of silver nitrate solution are added to the solution and a pale yellow precipitate forms. Halides precipitate with silver and sulfate precipitate with barium. Similar calculation for the leadII nitrate yields.
 Source: shutterstock.com
Source: shutterstock.com
Write the balanced equation for the reaction that took place between the salt and silver nitrate. In contrast many common ions give insoluble salts eg. A Nitrate Test is a chemical test used to determine the presence of nitrate ion in solution. No media kitchen no autoclave no bottles. Urea on the other hand has an NPK grade of 46-0-0 making it more economical to transport.

White lead is a basic lead carbonate PbCO 3 2 PbOH 2 which has been used as a white pigment because of its opaque quality though its use is now restricted because it can be a source for lead poisoning. White lead is a basic lead carbonate PbCO 3 2 PbOH 2 which has been used as a white pigment because of its opaque quality though its use is now restricted because it can be a source for lead poisoning. Ammonium nitrate N2H4O3 is one such compound and has an NPK rating of 34-0-0. Urea on the other hand has an NPK grade of 46-0-0 making it more economical to transport. Our purpose is to solve the toughest problems in life science by collaborating.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title solution of lead nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.