Source of pentane in combustion
Source Of Pentane In Combustion. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths. The other two are called isopentane methylbutane and neopentane dimethylpropane. The combustion of carbon compounds especially hydrocarbons has been the most important source of heat energy for human civilizations throughout recorded history. Isopentane is one of three structural isomers with the molecular formula C 5 H 12 the others being pentane n-pentane and neopentane dimethyl propane.
 Complete Combustion Of Pentane C5h12 Balanced Equation Youtube From youtube.com
Complete Combustion Of Pentane C5h12 Balanced Equation Youtube From youtube.com
The lower explosive limit LEL is the minimum concentration of a specific combustible gas required to fire combustion when in contact with oxygen air. Vapors heavier than air. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths. In order to test whether pentane excretion is related to age we measured breath pentane in 47 healthy subjects ages 21-79. Gasoline for internal combustion in engines in cars trucks and lawnmowers.
This liquid is used as an organic solvent transport fuels and cleansers.
The upper explosive limit UEL is the maximum level of concentration of the gas that will burn when mixed with oxygen. Isopentane is used in a closed loop in geothermal power production to drive turbines. Typically molar heat of combustion is given as kJmol so 297800217 1370. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths. Vapors heavier than air. Liquid hydrocarbons are rated in combustion properties relative to octane.
 Source: youtube.com
Source: youtube.com
We found that pentane excretion significantly P 005 r 032 increased with age. Pentane is an organic compound with the formula C 5 H 12 that is an alkane with five carbon atoms. Slightly larger hydrocarbon molecules known as kerosene jet fuel diesel fuel and oil for heating. The heat of combustion of 1 gram of ethanol equals -29782 J or 29780 kJ. Vapors heavier than air.
 Source: youtube.com
Source: youtube.com
Combustion Δ c H 298 33 MJ. Slightly denser than water and insoluble in water. In the IUPAC nomenclature however pentane means exclusively the n-pentane isomer. 1 g of ethanol is equal to 00217 moles. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths.
 Source: researchgate.net
Source: researchgate.net
Pentane 2-bromo-2-Pentyl bromide –2-Bromopentane. Gasoline for internal combustion in engines in cars trucks and lawnmowers. Typically molar heat of combustion is given as kJmol so 297800217 1370. 2-bromopentane appears as a colorless to yellow-colored liquid with a strong odor. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths.
 Source: energyeducation.ca
Source: energyeducation.ca
Typically molar heat of combustion is given as kJmol so 297800217 1370. The lower explosive limit LEL is the minimum concentration of a specific combustible gas required to fire combustion when in contact with oxygen air. The heat of combustion of 1 gram of ethanol equals -29782 J or 29780 kJ. We found that pentane excretion significantly P 005 r 032 increased with age. Typically molar heat of combustion is given as kJmol so 297800217 1370.
 Source: youtube.com
Source: youtube.com
The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths. 1 g of ethanol is equal to 00217 moles. If the concentration of the gas is below the LEL value the mix between the gas itself and the air is too weak to spark. Isopentane is one of three structural isomers with the molecular formula C 5 H 12 the others being pentane n-pentane and neopentane dimethyl propane. We found that pentane excretion significantly P 005 r 032 increased with age.
 Source: sciencedirect.com
Source: sciencedirect.com
The heat of combustion of 1 gram of ethanol equals -29782 J or 29780 kJ. 2-bromopentane appears as a colorless to yellow-colored liquid with a strong odor. The upper explosive limit UEL is the maximum level of concentration of the gas that will burn when mixed with oxygen. Isopentane is one of three structural isomers with the molecular formula C 5 H 12 the others being pentane n-pentane and neopentane dimethyl propane. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths.
 Source: researchgate.net
Source: researchgate.net
Cyclopentane is not an isomer of pentane. The term may refer to any of three structural isomers or to a mixture of them. In the IUPAC nomenclature however pentane means exclusively the n-pentane isomer. If the concentration of the gas is below the LEL value the mix between the gas itself and the air is too weak to spark. The practical importance of this reaction cannot be denied but the massive and uncontrolled chemical changes that take place in combustion make it difficult to deduce mechanistic paths.
 Source: en.wikipedia.org
Source: en.wikipedia.org
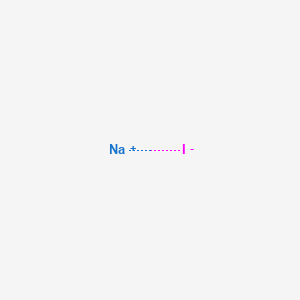
Using the combustion of propane as an example. We also measured serum levels of the antioxidants tocopherol retinol lycopene beta carotene ascorbate and zinc. Gasoline for internal combustion in engines in cars trucks and lawnmowers. The lower explosive limit LEL is the minimum concentration of a specific combustible gas required to fire combustion when in contact with oxygen air. The term may refer to any of three structural isomers or to a mixture of them.
 Source: youtube.com
Source: youtube.com
Using the combustion of propane as an example. Temperature and Pressure - Online calculator figures and table showing density and specific weight of pentane C 5 H 12 at temperatures ranging from -130 to 325 C -200 to 620 F at atmospheric and higher pressure - Imperial and SI Units. We found that pentane excretion significantly P 005 r 032 increased with age. The combustion of carbon compounds especially hydrocarbons has been the most important source of heat energy for human civilizations throughout recorded history. Of the six.
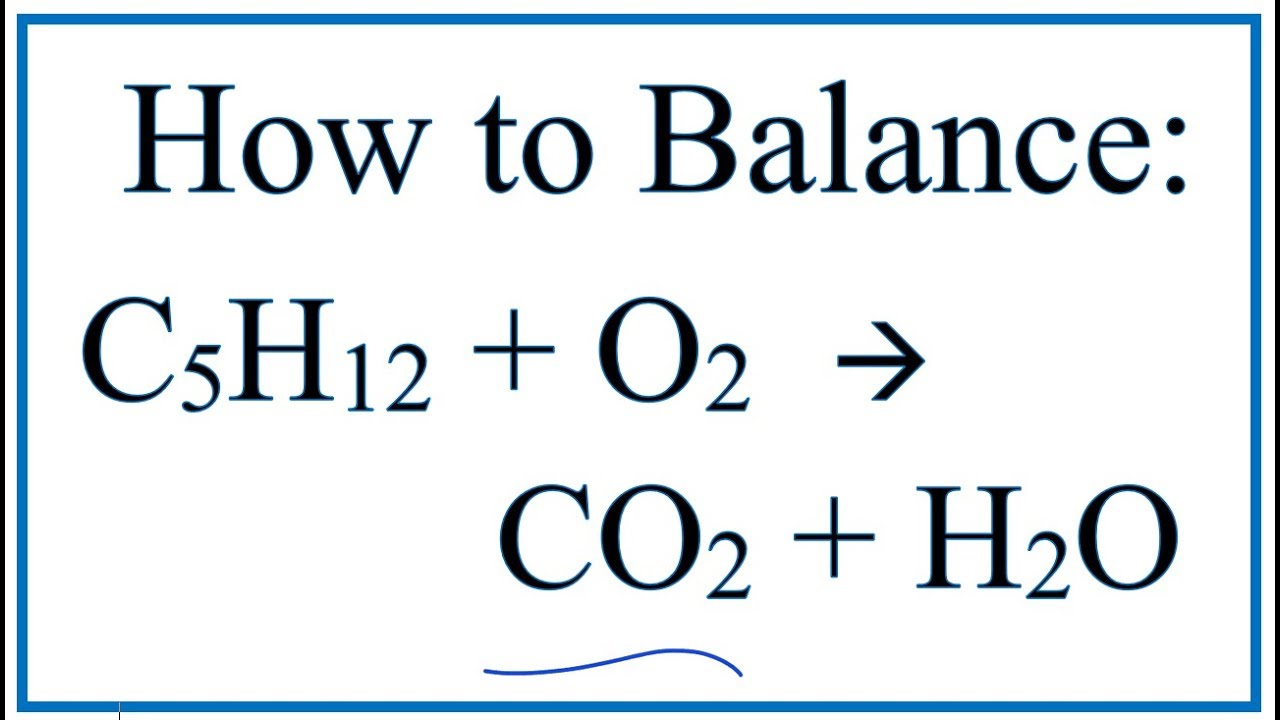 Source: youtube.com
Source: youtube.com
2-bromopentane appears as a colorless to yellow-colored liquid with a strong odor. Pentane - Density and Specific Weight vs. The upper explosive limit UEL is the maximum level of concentration of the gas that will burn when mixed with oxygen. Cyclopentane is not an isomer of pentane. Using the combustion of propane as an example.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title source of pentane in combustion by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.