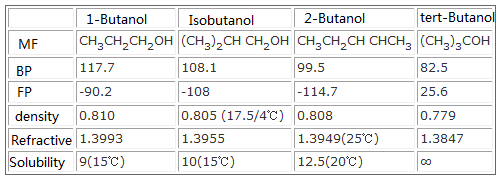
Special properties of 2 butanol
Special Properties Of 2 Butanol. N-butanol 2-butanol 23-butanediol 3-hydroxy-2-butanone and 23-butanedione. Samples from three individuals who died following LPG abuse contained a range of putative n-butane metabolites. Physical and Chemical Properties Physical State. However for the solvents water DMF and DCB this linear relationship was found to be poor.
 2 Butanol 78 92 2 From chemicalbook.com
2 Butanol 78 92 2 From chemicalbook.com
They have the same bonding but differ in the way that the. The pharmacokinetics of MEK in 70 male and female volunteers following a 4-hr inhalation exposure either alone 200 ppm or in combination with acetone 225 ppm 1125 ratio was described Blood and expired air elimination data for the MEK-exposed individuals revealed on average that MEK steady-state breath levels of about 10 to 12 ppm were attained non-steady-state peak blood levels of 3. The temperature is high C. Both left and right hand are chiral ky-ral and show chirality. Because the reaction occurs in one step it is concertedThe substrate and the nucleophile are both present in the transition state for this step. The pressure is low B.
They are nearly identical in their physical and chemical properties.
In fact enantiomers are so alike that they even share the same name. Both of the molecules are 2-butanol. Consequently it is very. The pharmacokinetics of MEK in 70 male and female volunteers following a 4-hr inhalation exposure either alone 200 ppm or in combination with acetone 225 ppm 1125 ratio was described Blood and expired air elimination data for the MEK-exposed individuals revealed on average that MEK steady-state breath levels of about 10 to 12 ppm were attained non-steady-state peak blood levels of 3. 168 mm Hg 25 deg C Vapor Density. 304 air1 Evaporation RateNot available.
 Source: chemicalbook.com
Source: chemicalbook.com
It is used to replace table sugar because it is half as energetic does not promote tooth decay and has a somewhat lesser effect on blood glucoseIn chemical terms maltitol is known as 4-O-α-glucopyranosyl-D-sorbitol. They have different melting points and boiling points and different densities. To the authors knowledge the last three compounds have not been proposed as metabolites of n-butane in man. Diastereomers can have different physical properties and reactivity. The pressure is high and the temperature is low D.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
They have the same bonding but differ in the way that the. The pharmacokinetics of MEK in 70 male and female volunteers following a 4-hr inhalation exposure either alone 200 ppm or in combination with acetone 225 ppm 1125 ratio was described Blood and expired air elimination data for the MEK-exposed individuals revealed on average that MEK steady-state breath levels of about 10 to 12 ppm were attained non-steady-state peak blood levels of 3. 2-Methyl-2-butanol none listed none listed none listed. The S N 2 mechanism is a one-step process in which a nucleophile attacks the substrate and a leaving group L departs simultaneously. The results of that study showed a linear relationship between MXene concentration and solvent viscosity for the solvents EtOH MeOH ACE ACN DMSO NMP PC HEX and TOL.
 Source: en.wikipedia.org
Source: en.wikipedia.org
In Figure 514 the two enantiomers of 2-butanol are shown. This is because of the special property of hand that is called chirality. The temperature is high C. Because the reaction occurs in one step it is concertedThe substrate and the nucleophile are both present in the transition state for this step. David Rawn in Organic Chemistry Second Edition 2018 The S N 2 Mechanism.

Samples from three individuals who died following LPG abuse contained a range of putative n-butane metabolites. Consequently it is very. In Figure 514 the two enantiomers of 2-butanol are shown. The pressure is high and the temperature is low D. The pressure is low B.
 Source: iea-amf.org
Source: iea-amf.org
Consequently it is very. In Figure 514 the two enantiomers of 2-butanol are shown. In fact enantiomers are so alike that they even share the same name. They have the same bonding but differ in the way that the. A special-purpose piece of laboratory glassware called pycnometer is used to measure liquid densities.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Both of the molecules are 2-butanol. Diastereomers can have different physical properties and reactivity. 2-Butanol or sec-butanol is an organic compound with formula C H 3 CHOHCH 2 CH 3This secondary alcohol is a flammable colorless liquid that is soluble in three parts water and completely miscible with organic solvents. They have the same bonding but differ in the way that the. In Figure 514 the two enantiomers of 2-butanol are shown.
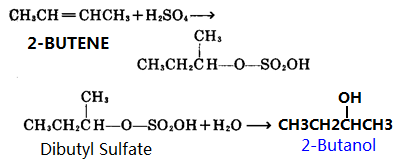 Source: chemicalbook.com
Source: chemicalbook.com
The perspective formula show the 3D structure of 2-butanol in two different ways and they are non-superimposable mirror images to each other. It has a volume of 25 cc. They have different melting points and boiling points and different densities. The two mirror images are different molecules. The pressure is low and the temperature is high.
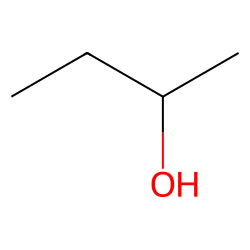 Source: chemeo.com
Source: chemeo.com
To the authors knowledge the last three compounds have not been proposed as metabolites of n-butane in man. David Rawn in Organic Chemistry Second Edition 2018 The S N 2 Mechanism. However for the solvents water DMF and DCB this linear relationship was found to be poor. 168 mm Hg 25 deg C Vapor Density. Camphor - sharp odor - unpleasant odor pH.
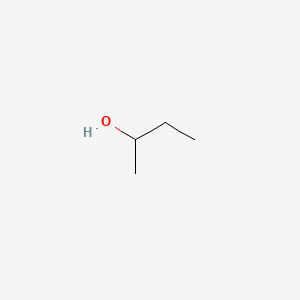
Consequently it is very. Both left and right hand are chiral ky-ral and show chirality. The viscoelastic properties of MXene dispersed in various solvents has already been studied. A special-purpose piece of laboratory glassware called pycnometer is used to measure liquid densities. They have the same bonding but differ in the way that the.
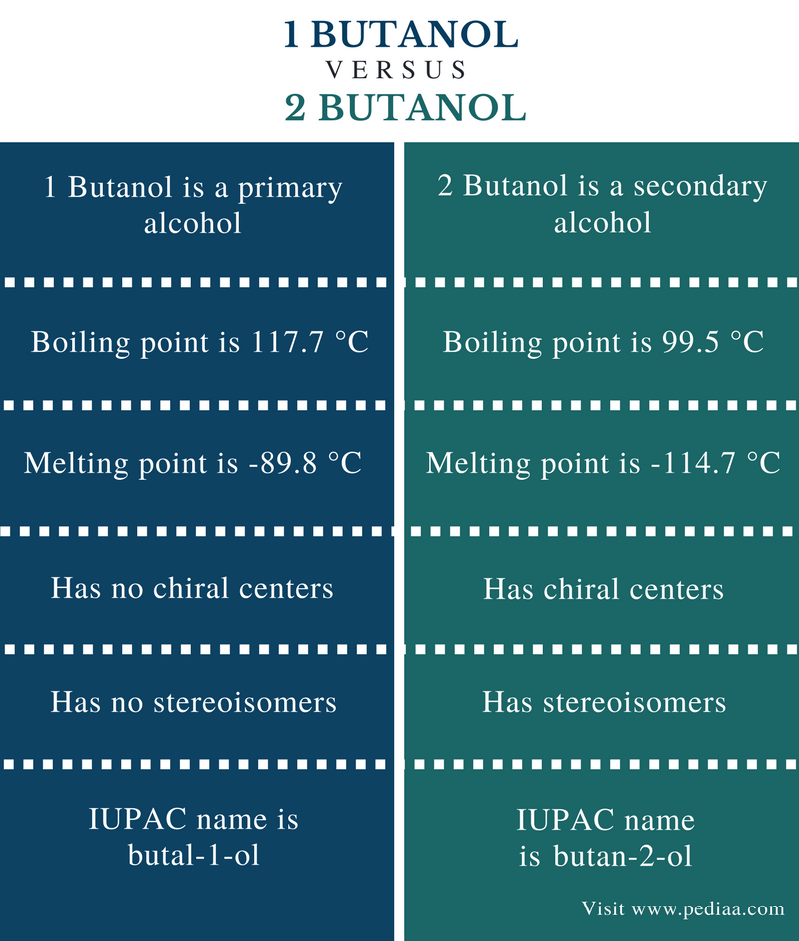 Source: pediaa.com
Source: pediaa.com
David Rawn in Organic Chemistry Second Edition 2018 The S N 2 Mechanism. Camphor - sharp odor - unpleasant odor pH. Diastereomers can have different physical properties and reactivity. To the authors knowledge the last three compounds have not been proposed as metabolites of n-butane in man. 168 mm Hg 25 deg C Vapor Density.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title special properties of 2 butanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





