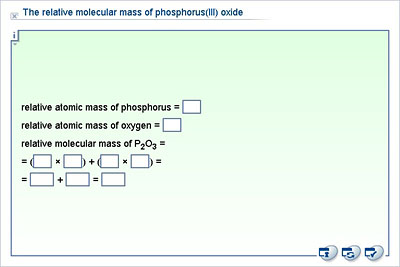
Sulfur trioxide solubility in water
Sulfur Trioxide Solubility In Water. It is the most potent greenhouse gas currently known with a global warming potential of 23900 times that of CO2 over a 100 year period SF6 has an estimated lifetime in the atmosphere of between 800 and 3000 years. May react explosively with maleic anhydride MCA Case History 622 1960. Combinations of finely divided sulfur with finely divided bromates chlorates or iodates of barium calcium magnesium potassium sodium or zinc can explode with heat friction percussion and sometimes light. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
 Is So3 Soluble In Water From byjus.com
Is So3 Soluble In Water From byjus.com
Sulfur is insoluble in water but soluble in carbon disulfide and to a lesser extent in other nonpolar organic solvents. Handled at elevated temperature typically 290F to prevent solidification and makes transfers easier. It has a role as an ultrasound contrast agent and a. Sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic. Reacts rapidly and exothermically with organic and inorganic acids with organic and inorganic acid anhydrides including oxides of nonmetals such as sulfur dioxide sulfur trioxide phosphorus trioxide phosphorus pentaoxide and with organic and inorganic acid chlorides. Sulfur trioxide made by catalysis from sulfur dioxide and sulfuric acid are similarly highly acidic and corrosive in the presence of water.
It has a role as an ultrasound contrast agent and a.
Handled at elevated temperature typically 290F to prevent solidification and makes transfers easier. May react explosively with maleic anhydride MCA Case History 622 1960. Sulfur trioxide alternative spelling sulphur trioxide also known as nisso sulfan is the chemical compound with the formula SO 3It has been described as unquestionably the most important economically sulfur oxide. Reacts violently with phosphorus trioxide Chem. Sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds.
 Source: slidetodoc.com
Source: slidetodoc.com
Transported as a yellow to red liquid. Sulfur trioxide alternative spelling sulphur trioxide also known as nisso sulfan is the chemical compound with the formula SO 3It has been described as unquestionably the most important economically sulfur oxide. Sulfur is insoluble in water but soluble in carbon disulfide and to a lesser extent in other nonpolar organic solvents. Sulfur trioxide exists in several forms - gaseous monomer crystalline trimer and solid polymer. Hot enough that plastic or rubber may melt or.
 Source: sciencemadness.org
Source: sciencemadness.org
Melting point - the temperature at which a solid turns into a liquid. Handled at elevated temperature typically 290F to prevent solidification and makes transfers easier. Boiling point - the temperature at which a liquid turns into a gas. Transported as a yellow to red liquid. These compounds can further oxidize and rain out as sulfuric or sulfurous acid.
 Source: byjus.com
Source: byjus.com
Sulfur trioxide alternative spelling sulphur trioxide also known as nisso sulfan is the chemical compound with the formula SO 3It has been described as unquestionably the most important economically sulfur oxide. It is the most potent greenhouse gas currently known with a global warming potential of 23900 times that of CO2 over a 100 year period SF6 has an estimated lifetime in the atmosphere of between 800 and 3000 years. Melting point - the temperature at which a solid turns into a liquid. It is prepared on an industrial scale as a precursor to sulfuric acid. Reacts violently with phosphorus trioxide Chem.
 Source: youtube.com
Source: youtube.com
Reacts rapidly and exothermically with organic and inorganic acids with organic and inorganic acid anhydrides including oxides of nonmetals such as sulfur dioxide sulfur trioxide phosphorus trioxide phosphorus pentaoxide and with organic and inorganic acid chlorides. This is the mechanism for acid rain which has reeked havoc on the forests of the northeastern United States as. Sulfur trioxide exists in several forms - gaseous monomer crystalline trimer and solid polymer. Transported as a yellow to red liquid. Mixtures with ammonium nitrate or with metal powders can be exploded by shock Kirk and Othmer 8644.
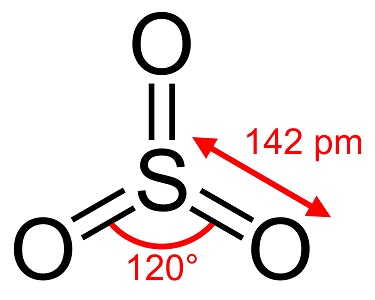 Source: chemicalbook.com
Source: chemicalbook.com
Transported as a yellow to red liquid. It has a role as an ultrasound contrast agent and a. Transported as a yellow to red liquid. When sulfur burns in air it generally forms sulfur dioxide or sulfur trioxide the latter of which lacks any smell amended from the podcast audio file which states that sulfur dioxide does not smell. Combinations of finely divided sulfur with finely divided bromates chlorates or iodates of barium calcium magnesium potassium sodium or zinc can explode with heat friction percussion and sometimes light.
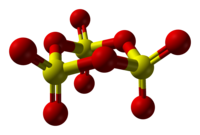 Source: en.wikipedia.org
Source: en.wikipedia.org
Transported as a yellow to red liquid. Sulfur molten appears as a pale yellow crystalline solid with a faint odor of rotten eggs. Sulfur trioxide alternative spelling sulphur trioxide also known as nisso sulfan is the chemical compound with the formula SO 3It has been described as unquestionably the most important economically sulfur oxide. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic.
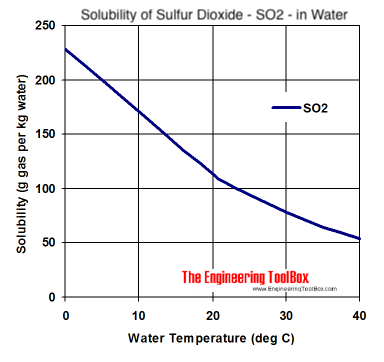 Source: engineeringtoolbox.com
Source: engineeringtoolbox.com
Sulfur hexafluoride is a sulfur coordination entity consisting of six fluorine atoms attached to a central sulfur atom. A fire and explosion risk above 450 F. Sulfuric acid is a strong dehydrating agent that can strip available water molecules and water components from sugar and organic. Reacts rapidly and exothermically with organic and inorganic acids with organic and inorganic acid anhydrides including oxides of nonmetals such as sulfur dioxide sulfur trioxide phosphorus trioxide phosphorus pentaoxide and with organic and inorganic acid chlorides. Reacts violently with phosphorus trioxide Chem.
 Source: sciencedirect.com
Source: sciencedirect.com
Mixtures with ammonium nitrate or with metal powders can be exploded by shock Kirk and Othmer 8644. Sulfur is insoluble in water but soluble in carbon disulfide and to a lesser extent in other nonpolar organic solvents. May react explosively with maleic anhydride MCA Case History 622 1960. Handled at elevated temperature typically 290F to prevent solidification and makes transfers easier. When sulfur burns in air it generally forms sulfur dioxide or sulfur trioxide the latter of which lacks any smell amended from the podcast audio file which states that sulfur dioxide does not smell.
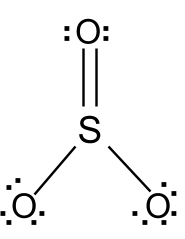 Source: protonstalk.com
Source: protonstalk.com
May react explosively with maleic anhydride MCA Case History 622 1960. See Standard state and enthalpy of formation Gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. A fire and explosion risk above 450 F. These compounds can further oxidize and rain out as sulfuric or sulfurous acid. Reacts rapidly and exothermically with organic and inorganic acids with organic and inorganic acid anhydrides including oxides of nonmetals such as sulfur dioxide sulfur trioxide phosphorus trioxide phosphorus pentaoxide and with organic and inorganic acid chlorides.
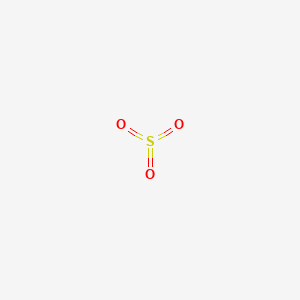
Mixtures with ammonium nitrate or with metal powders can be exploded by shock Kirk and Othmer 8644. It is prepared on an industrial scale as a precursor to sulfuric acid. It has a role as an ultrasound contrast agent and a. Reacts rapidly and exothermically with organic and inorganic acids with organic and inorganic acid anhydrides including oxides of nonmetals such as sulfur dioxide sulfur trioxide phosphorus trioxide phosphorus pentaoxide and with organic and inorganic acid chlorides. For full table with Density Liquid Denity at Melting Point and Water Solubility-rotate the screen.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title sulfur trioxide solubility in water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.