Tetrachloroethylene molar mass
Tetrachloroethylene Molar Mass. Academiaedu is a platform for academics to share research papers. With light aliphatic hydrocarbons such as pentane and hexane and with aliphatic chlorides such as trichloroethane and tetrachloroethylene. μ molar ionic strength. Tetrachloroethylene is mainly used as a cleaning solvent in dry cleaning and textile processing and in the manufacture of fluorocarbons.
 Tetrachloroethylene C2cl4 Coolgyan Org From coolgyan.org
Tetrachloroethylene C2cl4 Coolgyan Org From coolgyan.org
In Kh -2297T-1. In this study we systematically examine the relevancy of such an assumption with real-world data. μ molar ionic strength. 1051 c Because the helium atoms are of lower mass than the average air molecule the helium gas is less dense than air. Ethanols miscibility with water contrasts with the immiscibility of longer-chain alcohols five or more carbon atoms whose water miscibility decreases sharply as the. 146 gcm 3 at 20 C Melting point.
In this study we systematically examine the relevancy of such an assumption with real-world data.
It is a monocarboxylic acid and an organochlorine compound. Magnetic susceptibility χ 65810 6 cm 3 mol. It is a monocarboxylic acid and an organochlorine compound. In Kh -2297T-1. 1051 c Because the helium atoms are of lower mass than the average air molecule the helium gas is less dense than air. 13138 gmol Appearance Colorless liquid Odor.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Tetrachloroethylene is mainly used as a cleaning solvent in dry cleaning and textile processing and in the manufacture of fluorocarbons. Tetrachloroethylene is mainly used as a cleaning solvent in dry cleaning and textile processing and in the manufacture of fluorocarbons. 146 gcm 3 at 20 C Melting point. 58 mmHg 0076 atm at 20 C. In Kh -2297T-1.
 Source: acs.org
Source: acs.org
The balloon thus weighs less than the air displaced by its volume. Ethanols miscibility with water contrasts with the immiscibility of longer-chain alcohols five or more carbon atoms whose water miscibility decreases sharply as the. 13138 gmol Appearance Colorless liquid Odor. National Emission Inventory for 95. The balloon thus weighs less than the air displaced by its volume.
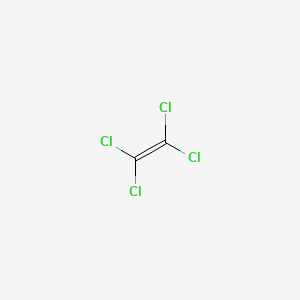
National Emission Inventory for 95. 1049 For gas samples at the same conditions molar mass determines density. Tetrachloroethylene is mainly used as a cleaning solvent in dry cleaning and textile processing and in the manufacture of fluorocarbons. 58 mmHg 0076 atm at 20 C. Tetrachloroethylene is a colorless volatile nonflammable liquid chlorinated hydrocarbon with an ether-like odor that may emit toxic fumes of phosgene when exposed to sunlight or flames.
 Source: en.wiktionary.org
Source: en.wiktionary.org
13138 gmol Appearance Colorless liquid Odor. Ethanols miscibility with water contrasts with the immiscibility of longer-chain alcohols five or more carbon atoms whose water miscibility decreases sharply as the. The following equation predicts all the measured values of Xh with a root mean square deviation in KhobsdKhcalcd of 23 T temperature in Kelvin. 46069 gmol 1 Appearance Colourless liquid Odor. 1883 K Boiling point.

1049 For gas samples at the same conditions molar mass determines density. 1051 c Because the helium atoms are of lower mass than the average air molecule the helium gas is less dense than air. Ethanols miscibility with water contrasts with the immiscibility of longer-chain alcohols five or more carbon atoms whose water miscibility decreases sharply as the. It is a monocarboxylic acid and an organochlorine compound. We used the reported emission data as background emissions from the 2017 US.

National Emission Inventory for 95. Of the three gases listed c Cl 2 has the largest molar mass. 1051 c Because the helium atoms are of lower mass than the average air molecule the helium gas is less dense than air. The balloon thus weighs less than the air displaced by its volume. In Kh -2297T-1.
 Source: waterfilteradvisor.com
Source: waterfilteradvisor.com
872 C 1890 F. Of the three gases listed c Cl 2 has the largest molar mass. μ molar ionic strength. The following equation predicts all the measured values of Xh with a root mean square deviation in KhobsdKhcalcd of 23 T temperature in Kelvin. Magnetic susceptibility χ 65810 6 cm 3 mol.
 Source: coolgyan.org
Source: coolgyan.org
Magnetic susceptibility χ 65810 6 cm 3 mol. Tetrachloroethylene is a colorless volatile nonflammable liquid chlorinated hydrocarbon with an ether-like odor that may emit toxic fumes of phosgene when exposed to sunlight or flames. It is a monocarboxylic acid and an organochlorine compound. Magnetic susceptibility χ 65810 6 cm 3 mol. Academiaedu is a platform for academics to share research papers.

It is a monocarboxylic acid and an organochlorine compound. Magnetic susceptibility χ 65810 6 cm 3 mol. 46069 gmol 1 Appearance Colourless liquid Odor. 1049 For gas samples at the same conditions molar mass determines density. Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5.
 Source: en.wikipedia.org
Source: en.wikipedia.org
μ molar ionic strength. 3603 K Solubility in water. The following equation predicts all the measured values of Xh with a root mean square deviation in KhobsdKhcalcd of 23 T temperature in Kelvin. 1883 K Boiling point. Academiaedu is a platform for academics to share research papers.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title tetrachloroethylene molar mass by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.