Thiazole structure with 2 nitrogens
Thiazole Structure With 2 Nitrogens. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one. For LTA4H-h six pharmacophore centers were identified that. Academiaedu is a platform for academics to share research papers. There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11.
 Structure Of The Investigated Thiazole Derivatives Download Scientific Diagram From researchgate.net
Structure Of The Investigated Thiazole Derivatives Download Scientific Diagram From researchgate.net
The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets. Finally examples 4 through 7 illustrate reactions of 12- and 13-oxazole thiazole and diazole. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one. When the Execute p1 button is clicked the javascript function p1 is executed. Quantum mechanical QM calculations imply that the pyrimidn-4-3H-ones 8 Tauto-1 and 10. For LTA4H-h six pharmacophore centers were identified that.
History and use in industry.
There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. History and use in industry. There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets. Quantum mechanical QM calculations imply that the pyrimidn-4-3H-ones 8 Tauto-1 and 10. Academiaedu is a platform for academics to share research papers.
 Source: researchgate.net
Source: researchgate.net
For LTA4H-h six pharmacophore centers were identified that. They were originally used and still are to increase the sensitivity range of photographic emulsions ie to increase the range of wavelengths which. Both nitrogens may each be independently part of a heteroaromatic moiety such as pyrrole imidazole thiazole pyridine quinoline indole benzothiazole etc. There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. Quantum mechanical QM calculations imply that the pyrimidn-4-3H-ones 8 Tauto-1 and 10.
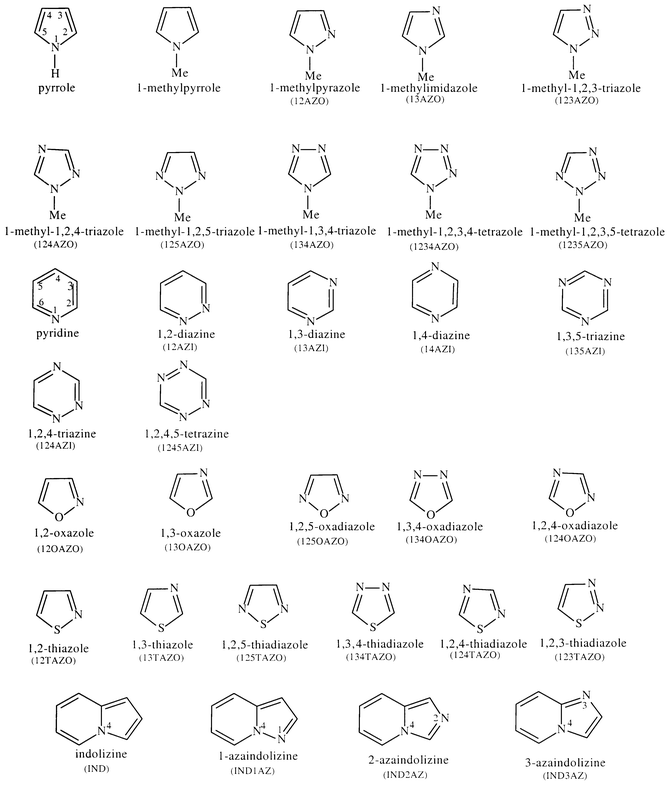 Source: pubs.rsc.org
Source: pubs.rsc.org
Quantum mechanical QM calculations imply that the pyrimidn-4-3H-ones 8 Tauto-1 and 10. They were originally used and still are to increase the sensitivity range of photographic emulsions ie to increase the range of wavelengths which. Both nitrogens may each be independently part of a heteroaromatic moiety such as pyrrole imidazole thiazole pyridine quinoline indole benzothiazole etc. In order to overcome limitations such as sub-optimal spectra. The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets.
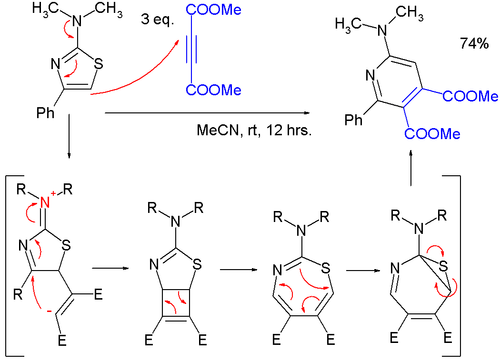 Source: en.wikipedia.org
Source: en.wikipedia.org
The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets. There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. Finally examples 4 through 7 illustrate reactions of 12- and 13-oxazole thiazole and diazole. Quantum mechanical QM calculations imply that the pyrimidn-4-3H-ones 8 Tauto-1 and 10. The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets.
 Source: researchgate.net
Source: researchgate.net
History and use in industry. The search for antifungal agents with acceptable toxicity profiles led first to the discovery of ketoconazole the first azole-based oral treatment of systemic fungal infections in the early 1980sLater triazoles fluconazole and itraconazole with a broader spectrum of antifungal activity and improved safety profile were developed. Both nitrogens may each be independently part of a heteroaromatic moiety such as pyrrole imidazole thiazole pyridine quinoline indole benzothiazole etc. When the Execute p1 button is clicked the javascript function p1 is executed. Finally examples 4 through 7 illustrate reactions of 12- and 13-oxazole thiazole and diazole.
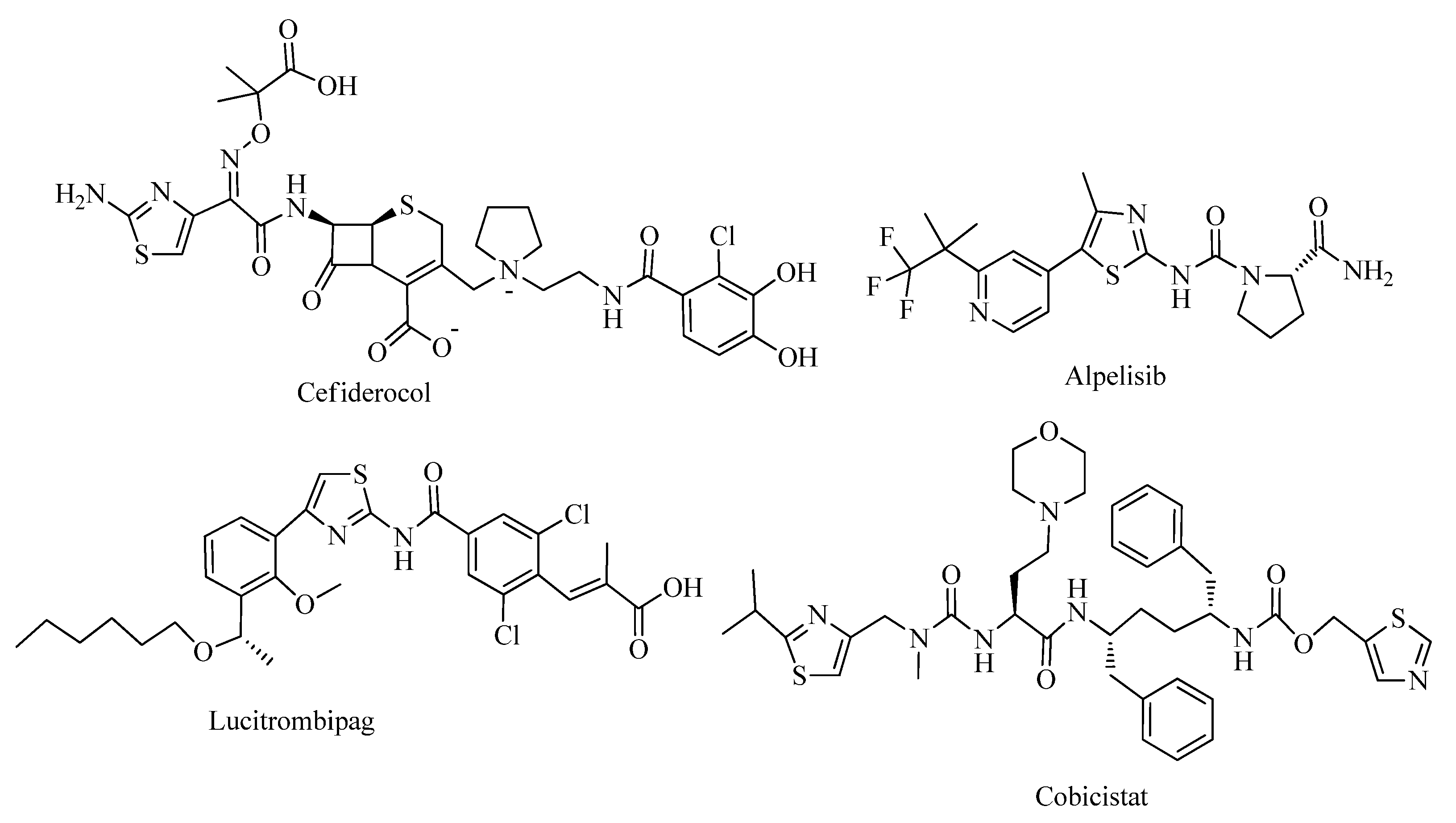 Source: mdpi.com
Source: mdpi.com
There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. History and use in industry. When the Execute p1 button is clicked the javascript function p1 is executed. The search for antifungal agents with acceptable toxicity profiles led first to the discovery of ketoconazole the first azole-based oral treatment of systemic fungal infections in the early 1980sLater triazoles fluconazole and itraconazole with a broader spectrum of antifungal activity and improved safety profile were developed. The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets.
 Source: researchgate.net
Source: researchgate.net
They were originally used and still are to increase the sensitivity range of photographic emulsions ie to increase the range of wavelengths which. Quantum mechanical QM calculations imply that the pyrimidn-4-3H-ones 8 Tauto-1 and 10. When the Execute p1 button is clicked the javascript function p1 is executed. Cyanines were first synthesized over a century ago. There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11.
 Source: britannica.com
Source: britannica.com
There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. In order to overcome limitations such as sub-optimal spectra. Finally examples 4 through 7 illustrate reactions of 12- and 13-oxazole thiazole and diazole. For LTA4H-h six pharmacophore centers were identified that. History and use in industry.
 Source: sciencedirect.com
Source: sciencedirect.com
For LTA4H-h six pharmacophore centers were identified that. Note that the basicity of the sp 2-hybridized nitrogen in the diazoles is over a million times greater than that of the apparent sp 3-hybridized nitrogen the electron pair of which is part of the aromatic electron sextet. When the Execute p1 button is clicked the javascript function p1 is executed. The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one.
 Source: researchgate.net
Source: researchgate.net
There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. Both nitrogens may each be independently part of a heteroaromatic moiety such as pyrrole imidazole thiazole pyridine quinoline indole benzothiazole etc. There are three tautomers of 8 and 10 in which the readily exchangeable hydrogen can be either on the pyrimidine nitrogens tautomers 8 Tauto-1 8 Tauto-2 10 Tauto-1 and 10 Tauto-2 Figure S11 or on the oxygen atom at the 4 position of the pyrimidine ring 8 Tauto-3 and 10 Tauto-3 Figure S11. Finally examples 4 through 7 illustrate reactions of 12- and 13-oxazole thiazole and diazole. History and use in industry.
 Source: en.wikipedia.org
Source: en.wikipedia.org
The cocrystal structure PDB code 1HS6 of LTA4H-h with 2-3-amino-2-hydroxy- 4-phenylbutyrylamino-4-methyl-pentanoic acid bestatin and the structure PDB code 1DB4 of PLA 2 with 3-1-benzyl-3-carbamoylmethyl-2-methyl-1H-indol-5-yloxypropylphosphonic acid indole 8 were used to derive pharmacophores of the two targets. 50 pointsThe textarea shown to the left is named ta in a form named f1It contains the top 10000 passwords in order of frequency of use – each followed by a comma except the last one. The search for antifungal agents with acceptable toxicity profiles led first to the discovery of ketoconazole the first azole-based oral treatment of systemic fungal infections in the early 1980sLater triazoles fluconazole and itraconazole with a broader spectrum of antifungal activity and improved safety profile were developed. Both nitrogens may each be independently part of a heteroaromatic moiety such as pyrrole imidazole thiazole pyridine quinoline indole benzothiazole etc. In order to overcome limitations such as sub-optimal spectra.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title thiazole structure with 2 nitrogens by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.




