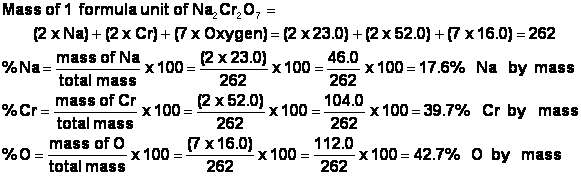Tin 4 nitrate
Tin 4 Nitrate. PbBr 4 - LeadIV Bromide. SO 3 2. 16-day-old vegetable amaranth Amaranthus iricolor L plants were transplanted 24 July 1986 and harvested 27 days later. K 3 P - Potassoum Phosphide.
 Tin Iv Nitrate American Elements From americanelements.com
Tin Iv Nitrate American Elements From americanelements.com
Zinc nitrate is usually prepared by dissolving zinc in nitric acid this reaction is concentration dependent with a reaction in. Ions grouped by charge anions grouped by periodic position Simple ions. Cs 3 P - Cesium Phosphide. Li 3 P - Lithium Phosphide. So if there is Pb 2 and then chloride Cl-ions in significant amounts the addition of AgNO 3 will precipitate AgCl white. H 2 PO 4.
Cu2CO3 Section B Write the formula of the ionic compounds containing polyatomic ions 1.
K 3 N - Potassium Nitride. Strontium nitrate is an inorganic compound composed of the elements strontium nitrogen and oxygen with the formula SrNO 3 2. It is soluble in both water and alcohol. Cadmium phosphate Cd3PO42 9. Li 3 N - Lithium Nitride. H 2 PO 4.
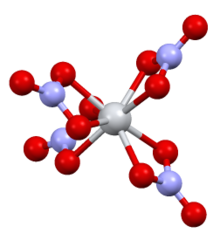 Source: en.wikipedia.org
Source: en.wikipedia.org
This colorless solid is used as a red colorant and oxidizer in pyrotechnics. HC 2 H 3 O 2 - Hydrogen Acetate. Chemistry HS Background. The washing water that we examine is added with some drops of silver nitrate AgNO 3. Mixtures of MAGNESIUM NITRATE with alkyl esters may explode owing to the formation of alkyl nitrates.
 Source: chemsrc.com
Source: chemsrc.com
PbBr 4 - LeadIV Bromide. PbBr 4 - LeadIV Bromide. This colorless solid is used as a red colorant and oxidizer in pyrotechnics. Cs 3 N - Cesium Nitride. SnOH2 tin II hydroxide 28.
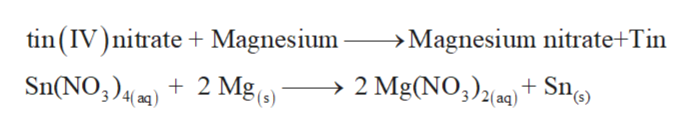 Source: bartleby.com
Source: bartleby.com
Strontium nitrate is an inorganic compound composed of the elements strontium nitrogen and oxygen with the formula SrNO 3 2. So if there is Pb 2 and then chloride Cl-ions in significant amounts the addition of AgNO 3 will precipitate AgCl white. K 3 N - Potassium Nitride. SO 3 2. Na 3 P - Sodium Phosphide.
 Source: chemicalbook.com
Source: chemicalbook.com
Li 3 N - Lithium Nitride. If large quantities are involved in a fire or the combustible material is finely divided an. Two levels of phosphorus 20 and 80 kg Pha and 2 levels of aluminum 0 and 85 kg Alha as aluminum nitrate were applied to Enders soils of pH 44 66 or 73. PbBr 4 - LeadIV Bromide. H 2 PO 4-Sulfate.
 Source: americanelements.com
Source: americanelements.com
Li 3 N - Lithium Nitride. The washing water that we examine is added with some drops of silver nitrate AgNO 3. Copper I sulfite Cu2SO3 8. Lead II chlorate PbClO32 2. Na 3 P - Sodium Phosphide.
 Source: youtube.com
Source: youtube.com
If large quantities are involved in a fire or the combustible material is finely divided an. Mixtures of MAGNESIUM NITRATE with alkyl esters may explode owing to the formation of alkyl nitrates. This colorless solid is used as a red colorant and oxidizer in pyrotechnics. If large quantities are involved in a fire or the combustible material is finely divided an. SO 3 2.
 Source: youtube.com
Source: youtube.com
Chemistry HS Background. 16-day-old vegetable amaranth Amaranthus iricolor L plants were transplanted 24 July 1986 and harvested 27 days later. Li 3 P - Lithium Phosphide. Cs 3 P - Cesium Phosphide. H 2 PO 4-Sulfate.
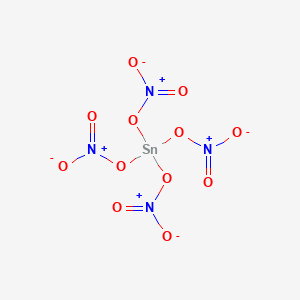
SnI 4 - TinIV Iodide. 16-day-old vegetable amaranth Amaranthus iricolor L plants were transplanted 24 July 1986 and harvested 27 days later. Zinc nitrate is usually prepared by dissolving zinc in nitric acid this reaction is concentration dependent with a reaction in. Ammonium cyanide NH4CN 5. K 3 P - Potassoum Phosphide.
 Source: youtube.com
Source: youtube.com
Cs 3 N - Cesium Nitride. Physics National 34. H 2 PO 4-Sulfate. Cs 3 P - Cesium Phosphide. Ammonium cyanide NH4CN 5.
 Source: youtube.com
Source: youtube.com
Strontium acetate SrC2H3O22 3. Zinc nitrate is usually prepared by dissolving zinc in nitric acid this reaction is concentration dependent with a reaction in. Mixtures with phosphorus tinII chloride or other reducing agents may react explosively Bretherick 1979 p. It is soluble in both water and alcohol. SnI 4 - TinIV Iodide.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title tin 4 nitrate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.