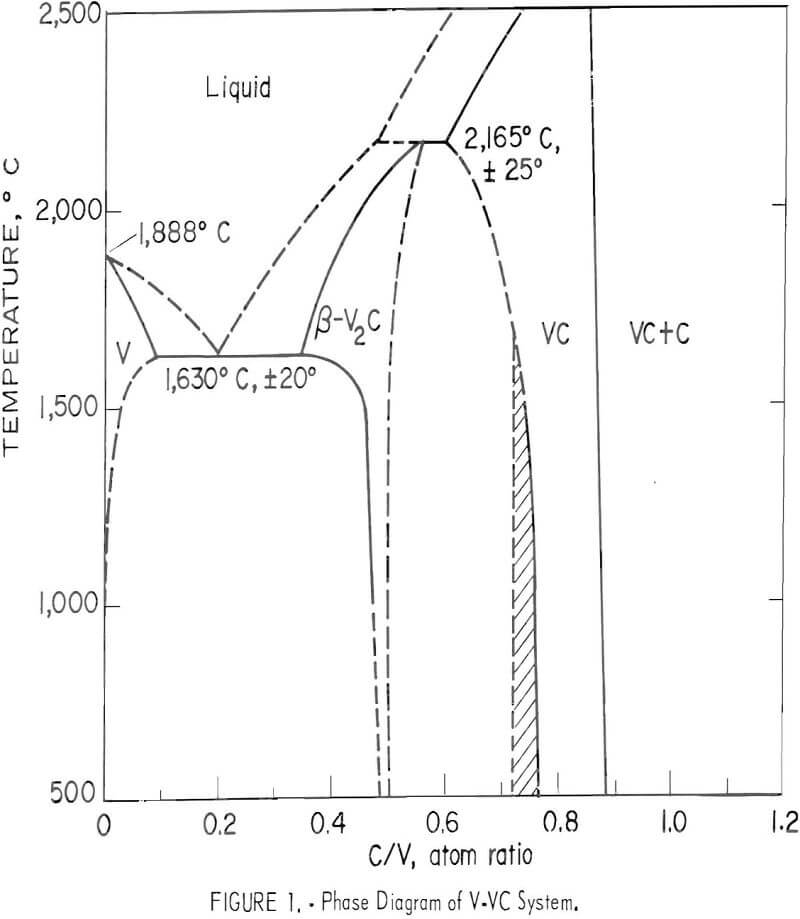
Tin carbide formula
Tin Carbide Formula. Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element. For the S- P-blocks. Metals from group 11 also tend to form acetylides such as copperI acetylide and silver acetylide. Tungsten Carbide WC 450 - 650.
 Titanium Carbide Nanopowder 200 Nm Particle Size Tem 12070 08 5 From sigmaaldrich.com
Titanium Carbide Nanopowder 200 Nm Particle Size Tem 12070 08 5 From sigmaaldrich.com
For the S- P-blocks. Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element. Metals from group 11 also tend to form acetylides such as copperI acetylide and silver acetylide. 1 GPa 10 9 Nm 2 10 6 Ncm 2 10 3 Nmm 2 0145x10 6 psi lb f in 2. TinII Sn 4 tinIV Cu copperI Cu 2 copperII Polyatomic positive ions often have common names ending with the suffix -onium. Silicon carbide SiC also known as carborundum ˌ k ɑːr b ə ˈ r ʌ n d əm is a semiconductor containing silicon and carbonIt occurs in nature as the extremely rare mineral moissaniteSynthetic SiC powder has been mass-produced since 1893 for use as an abrasiveGrains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in.
Metals from group 11 also tend to form acetylides such as copperI acetylide and silver acetylide.
Chemical Formula - Indicates the number and type of atoms in the base unit of a compound. 1 Pa Nm 2 1x10-6 Nmm 2 14504x10-4 psi. Sulfuric acid 98072 g. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Chemical Formula - Indicates the number and type of atoms in the base unit of a compound. Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element.
 Source: en.wikipedia.org
Source: en.wikipedia.org
H 3 O hydronium. NH 4 ammonium. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Negative ions that consist of a single atom are named by adding the suffix -ide to the stem of the name of the element. 2 carbide C2O4 2.
 Source: youtube.com
Source: youtube.com
Lanthanides also form carbides sesquicarbides see below with formula M 2 C 3. Carbides of the actinide elements which have stoichiometry MC 2 and M 2 C 3 are also described as salt-like derivatives. NH 4 ammonium. Silicon carbide SiC also known as carborundum ˌ k ɑːr b ə ˈ r ʌ n d əm is a semiconductor containing silicon and carbonIt occurs in nature as the extremely rare mineral moissaniteSynthetic SiC powder has been mass-produced since 1893 for use as an abrasiveGrains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in. Valence e Group number.
 Source: favpng.com
Source: favpng.com
Lanthanides also form carbides sesquicarbides see below with formula M 2 C 3. NH 4 ammonium. There exists however a mixed titanium-tin carbide which is a two-dimensional conductor. 1 GPa 10 9 Nm 2 10 6 Ncm 2 10 3 Nmm 2 0145x10 6 psi lb f in 2. H 3 O hydronium.
 Source: britannica.com
Source: britannica.com
For the S- P-blocks. There exists however a mixed titanium-tin carbide which is a two-dimensional conductor. Tungsten Carbide WC 450 - 650. Valence e Group number. 1 MPa 10 6 Pa Nm 2 0145x10 3 psi lb f in 2 0145 ksi.
 Source: britannica.com
Source: britannica.com
Metals from group 11 also tend to form acetylides such as copperI acetylide and silver acetylide. Silicon carbide SiC also known as carborundum ˌ k ɑːr b ə ˈ r ʌ n d əm is a semiconductor containing silicon and carbonIt occurs in nature as the extremely rare mineral moissaniteSynthetic SiC powder has been mass-produced since 1893 for use as an abrasiveGrains of silicon carbide can be bonded together by sintering to form very hard ceramics that are widely used in. 1 GPa 10 9 Nm 2 10 6 Ncm 2 10 3 Nmm 2 0145x10 6 psi lb f in 2. NH 4 ammonium. Carbides of the actinide elements which have stoichiometry MC 2 and M 2 C 3 are also described as salt-like derivatives.

Lanthanides also form carbides sesquicarbides see below with formula M 2 C 3. For the S- P-blocks. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Chemical Formula - Indicates the number and type of atoms in the base unit of a compound. Carbides of the actinide elements which have stoichiometry MC 2 and M 2 C 3 are also described as salt-like derivatives.
 Source: youtube.com
Source: youtube.com
Carbides of the actinide elements which have stoichiometry MC 2 and M 2 C 3 are also described as salt-like derivatives. H 3 O hydronium. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Carbides of the actinide elements which have stoichiometry MC 2 and M 2 C 3 are also described as salt-like derivatives. 1 MPa 10 6 Pa Nm 2 0145x10 3 psi lb f in 2 0145 ksi.
 Source: youtube.com
Source: youtube.com
TinII Sn 4 tinIV Cu copperI Cu 2 copperII Polyatomic positive ions often have common names ending with the suffix -onium. 190 - 210. H 3 O hydronium. Tungsten W 400 - 410. 2 carbide C2O4 2.
 Source: slideplayer.com
Source: slideplayer.com
Sulfuric acid 98072 g. For the S- P-blocks. TinII Sn 4 tinIV Cu copperI Cu 2 copperII Polyatomic positive ions often have common names ending with the suffix -onium. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. Type of compound Base unit Ionic Formula unit fu Molecular Molecule Valence Electrons - Electrons in the outermost shell of an atom The only e s involved in bonding and chemical reactions.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
190 - 210. Tungsten W 400 - 410. For the S- P-blocks. TinII Sn 4 tinIV Cu copperI Cu 2 copperII Polyatomic positive ions often have common names ending with the suffix -onium. 2 carbide C2O4 2.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title tin carbide formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.