Tin chloride formula
Tin Chloride Formula. The chemical formula is as follows. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. TnCl 4 5H 2 O. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A.
 Tin Iv Chloride Pentahydrate 98 10026 06 9 From sigmaaldrich.com
Tin Iv Chloride Pentahydrate 98 10026 06 9 From sigmaaldrich.com
MnBrO 2 2 4H 2 O. MgBr 2 4H 2 O. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. E-mail to a friend. Copy this to my account. The names are found by finding the intersection between the cations and anions.
MnBr 2 4H 2 O.
Ionic Compound Naming and Formula Writing List 1. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions. It is soluble in cold water and decomposed by hot water to form hydrochloric acid with the evolution. What is the correct. SnCl 4 5H 2 O. SnCl 2 5H 2 O.

MnBr 2 3H 2 O. E-mail to a friend. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown. Stannic chloride anhydrous is a colorless fuming liquid with a pungent odor. The calculator can be used to calculate the chemical formula of a range of 1.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
MnBr 2 4H 2 O. It is a white powdery solid. Ionic Compounds Naming and Formula Writing. MgBr 2 4H 2 O. Burns but may be difficult to ignite.

SnCl 2 5H 2 O. It can be made in pure form by burning. For example FeCl 2which would have been named iron2-chloride according to Stocks original idea became ironII chloride in the revised proposal. Stannic chloride anhydrous is a colorless fuming liquid with a pungent odor. MnBrO 2 2 4H 2 O.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Density 395 g cm3. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions. The names are found by finding the intersection between the cations and anions. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central. Ionic Compounds Naming and Formula Writing.
 Source: coolgyan.org
Source: coolgyan.org
When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions. SnCl 2 or Cl 2 Sn. MnBr 2 4H 2 O. Stannous chloride solid appears as crystalline mass or flaky solid with a fatty appearance. SnClO 2 2 5H 2 O.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
TinII chloride also known as stannous chloride is a white crystalline solid with the formula The template Tin is being considered for deletion Sn The template Chlorine is being considered for deletion Cl 2It forms a stable dihydrate but aqueous solutions tend to undergo hydrolysis particularly if hotSnCl 2 is widely used as a reducing agent in acid solution and in. It reacts with carbon at a high temperature to make tin metal. Copy this to my account. MnBr 4 4H 2 O. Pyridinium chloride can be produced by passing hydrogen chloride in pyridine dissolved in diethyl ether.
 Source: cs.mcgill.ca
Source: cs.mcgill.ca
SnCl 2 5H 2 O. MnBr 2 4H 2 O. What is the correct. Copy this to my account. The first box is the intersection between the zinc cation and the chloride anion so you should write ZnCl 2 as shown.
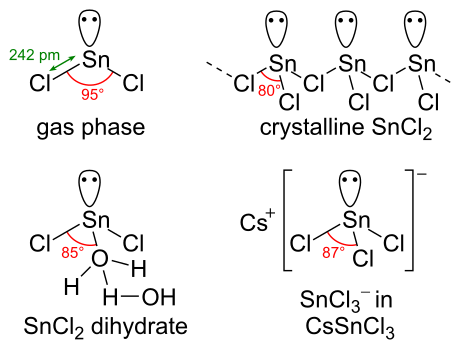 Source: en.wikipedia.org
Source: en.wikipedia.org
E-mail to a friend. SnClO 2 2 5H 2 O. Copy this to my account. SnCl 2 5H 2 O. Ionic Compound Naming and Formula Writing List 1.
 Source: youtube.com
Source: youtube.com
In 1934 Stock approved of the Roman numerals but felt it better to keep the hyphen and drop the parenthesis. When the formula unit contains two or more of the same polyatomic ion that ion is written within parentheses and a subscript is written outside the parentheses to indicate the number of polyatomic ions. An ionic compound is composed of a metal and a non. Cl 4 Sn or SnCl 4. It is soluble in cold water and decomposed by hot water to form hydrochloric acid with the evolution.
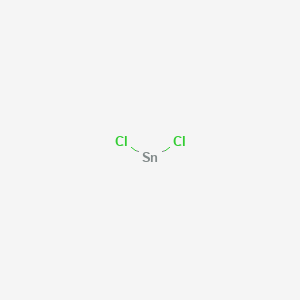
MnBr 2 4H 2 O. SnCl 2 5H 2 O. TnCl 4 5H 2 O. The names are found by finding the intersection between the cations and anions. E-mail to a friend.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title tin chloride formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.