Tin ii fluoride
Tin Ii Fluoride. H 2 PO 4-Sulfate. S and O-2-Ethylthioethyl OO-dimethyl phosphorothioate. Aluminium fluoride refers to inorganic compounds with the formula AlF 3 xH 2 O. With the formula AlF 3 xH 2 O these compounds include monohydrate x 1 two polymorphs of the trihydrate.
 How To Write The Formula For Tin Ii Fluoride Youtube From youtube.com
How To Write The Formula For Tin Ii Fluoride Youtube From youtube.com
Magnesium ammonium phosphate MgNH 4 PO 4 2510 13. The tin fluoride is used as addictive in toothpastes. Lead IV chloride. Sn 4 tinIV ion. Almost no odor in pure state. Strontium bromide SrBr2 44.
Bi 3 bismuthIII ion.
TIN II FLUORIDE Stannous fluoride. DEMETON MIXED ISOMERS 0862. Mercury II iodide HgI2 32. DEMETON-S OO-Diethyl S-2-ethylthioethyl phosphorothioate. Almost no odor in pure state. It has also been shown to be more effective than sodium fluoride in controlling gingivitis.
 Source: en.wikipedia.org
Source: en.wikipedia.org
Copper I phosphide Cu3P 46. Magnesium ammonium phosphate MgNH 4 PO 4 2510 13. Used as a quick-acting military chemical nerve agent. LeadII iodide PbI 2 7110 9. TinII fluoride can be mixed with calcium abrasives while the more common sodium fluoride gradually becomes biologically inactive in the presence of calcium compounds.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
They are all colorless solids. Aside from anhydrous AlF 3 several hydrates are known. TinII fluoride is added to some dental care products as stannous fluoride SnF 2. It has also been shown to be more effective than sodium fluoride in controlling gingivitis. LeadII iodide PbI 2 7110 9.
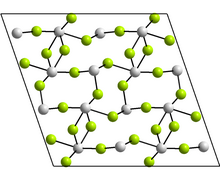 Source: en.wikipedia.org
Source: en.wikipedia.org
Lead IV chloride. Ions grouped by charge anions grouped by periodic position Simple ions. Bi 5 bismuthV ion. TinII fluoride can be mixed with calcium abrasives while the more common sodium fluoride gradually becomes biologically inactive in the presence of calcium compounds. 6 Hexa HF Hydrogen fluoride N 2O 2 Dinitrogen dioxide 7 Hepta Cl 2 Chlorine N 2O 4 Dinitrogen tetroxide 8 Octa HCl Hydrogen chloride CO Carbon monoxide 9 Nona Br 2 Bromine CO 2 Carbon dioxide 10 Deca I 2 Iodine CCl 4 Carbon tetrachloride Organic Nomenclature and Symbolism Other group prefixeslongest chain prefixhighest bond rootmost.
 Source: youtube.com
Source: youtube.com
Sarin appears as a colorless odorless liquid. LeadII iodide PbI 2 7110 9. Sn2 TinII stannous ion As3-Arsinide ion. TIN II FLUORIDE Stannous fluoride. Anhydrous AlF 3 is used in the production of aluminium metal.
 Source: gelest.com
Source: gelest.com
FeF 2 iron II fluoride Pb 2C lead II carbide NiC ℓ 2 nickel II chloride Sr 3P 2 strontium phosphide HgO mercury II oxide CuF copper I fluoride CoBr 3 cobalt III bromide NiBr 3 nickel III bromide CrS chromium II sulfide FeN iron III nitride NiN nickel III nitride SiO 2 silicon oxide SnO 2 tin IV oxide Sb 2S 5 antimony V sulfide Au 3P gold I phosphide AsH 3 arsenic. Mercury I iodide Hg2I2 40. Sn 4 tinIV ion. Almost no odor in pure state. Sn 2 tinII ion.
 Source: fishersci.co.uk
Source: fishersci.co.uk
Main-Group Nonmetals Groups IVA VA VIA and VIIA Group IVA VA VIA and VIIA nonmetals tend to form anions by gaining enough electrons to fill their valence shell with eight electrons. HPO 4 2-Dihydrogen phosphate. The more important tin compound is the tin dioxide SnO 2 used in electric resistors and dielectrics and the tin monoxide that it is used in the production of tin salts for electroplating and as chemical reagents. TIN II FLUORIDE Stannous fluoride. Sn 4 tinIV ion.
 Source: alibaba.com
Source: alibaba.com
The more important tin compound is the tin dioxide SnO 2 used in electric resistors and dielectrics and the tin monoxide that it is used in the production of tin salts for electroplating and as chemical reagents. HPO 4 2-Dihydrogen phosphate. Lithium fluoride LiF 3810 3. The more important tin compound is the tin dioxide SnO 2 used in electric resistors and dielectrics and the tin monoxide that it is used in the production of tin salts for electroplating and as chemical reagents. Sarin appears as a colorless odorless liquid.
Lithium phosphide Li3P 41. Sn2 TinII stannous ion As3-Arsinide ion. FeF 2 iron II fluoride Pb 2C lead II carbide NiC ℓ 2 nickel II chloride Sr 3P 2 strontium phosphide HgO mercury II oxide CuF copper I fluoride CoBr 3 cobalt III bromide NiBr 3 nickel III bromide CrS chromium II sulfide FeN iron III nitride NiN nickel III nitride SiO 2 silicon oxide SnO 2 tin IV oxide Sb 2S 5 antimony V sulfide Au 3P gold I phosphide AsH 3 arsenic. HPO 4 2-Dihydrogen phosphate. TinII fluoride can be mixed with calcium abrasives while the more common sodium fluoride gradually becomes biologically inactive in the presence of calcium compounds.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
Anhydrous AlF 3 is used in the production of aluminium metal. FeF 2 iron II fluoride Pb 2C lead II carbide NiC ℓ 2 nickel II chloride Sr 3P 2 strontium phosphide HgO mercury II oxide CuF copper I fluoride CoBr 3 cobalt III bromide NiBr 3 nickel III bromide CrS chromium II sulfide FeN iron III nitride NiN nickel III nitride SiO 2 silicon oxide SnO 2 tin IV oxide Sb 2S 5 antimony V sulfide Au 3P gold I phosphide AsH 3 arsenic. Lead IV chloride. Tin II sulfide SnS 31. Main-Group Nonmetals Groups IVA VA VIA and VIIA Group IVA VA VIA and VIIA nonmetals tend to form anions by gaining enough electrons to fill their valence shell with eight electrons.
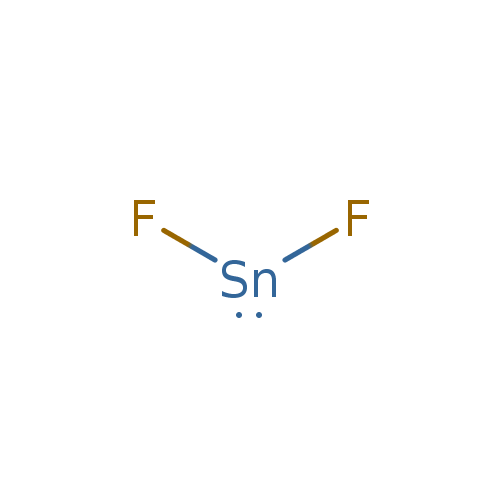 Source: t3db.ca
Source: t3db.ca
The more important tin compound is the tin dioxide SnO 2 used in electric resistors and dielectrics and the tin monoxide that it is used in the production of tin salts for electroplating and as chemical reagents. Aside from anhydrous AlF 3 several hydrates are known. LeadII fluoride PbF 2 2710 8. Several occur as minerals. Bi 5 bismuthV ion.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title tin ii fluoride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






