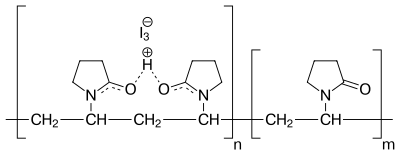
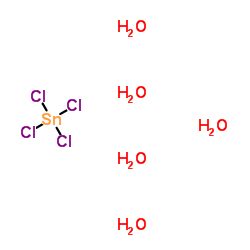
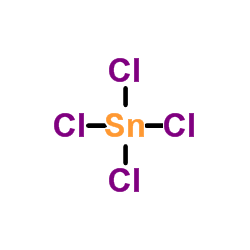
Tin iv chloride stability
Tin Iv Chloride Stability. TinIV chloride solution 10 M in methylene chloride. Stability of cubic tin sulphide nanocrystals. The physical and cellular mechanism of nano-crystals based color change in zebrafish. In the absence of interfering substances the precision and accuracy are estimated to be about 012 mg for 5 mg chloride or 25 of the amount present.
 Tin Iv Chloride Wikipedia From en.wikipedia.org
Tin Iv Chloride Wikipedia From en.wikipedia.org
TinIV chloride 1M solution in heptane AcroSealR PubChem. It has a tetrahedral configuration with lead as the central atom. The monthly publication features timely original peer-reviewed articles on the newest techniques dental materials and research findings. Thionyl chloride is primarily used as a chlorinating reagent with approximately 45000 tonnes 50000 short tons per year being produced during the early 1990s but is occasionally also used as a solvent. 3 Chemical and Physical Properties. Dvir Gur Weizmann Institute of Science 1225-1245.
Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count.
An Operando Kelvin Probe Force. 3 Chemical and Physical Properties. It is toxic reacts with water. It is a moderately volatile colourless liquid with an unpleasant acrid odour. Property Name Property Value Reference. Observing Electrochemical Reactions on Suspended Graphene.
 Source: wikiwand.com
Source: wikiwand.com
Observing Electrochemical Reactions on Suspended Graphene. It is toxic reacts with water. Stability of cubic tin sulphide nanocrystals. Observing Electrochemical Reactions on Suspended Graphene. Role of ammonium chloride surfactant headgroups Ran Eitan Abutbul Ben-Gurion University of the Negev.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It has a tetrahedral configuration with lead as the central atom. The PbCl covalent bonds have been. Dvir Gur Weizmann Institute of Science 1225-1245. Property Name Property Value Reference. Thionyl chloride is primarily used as a chlorinating reagent with approximately 45000 tonnes 50000 short tons per year being produced during the early 1990s but is occasionally also used as a solvent.

It is a moderately volatile colourless liquid with an unpleasant acrid odour. Dvir Gur Weizmann Institute of Science 1225-1245. The PbCl covalent bonds have been. An Operando Kelvin Probe Force. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count.
 Source: wikiwand.com
Source: wikiwand.com
Chloride is determined by potentiometric titration with silver nitrate solution with a glass and silver-silver chloride electrode system. Thionyl chloride is primarily used as a chlorinating reagent with approximately 45000 tonnes 50000 short tons per year being produced during the early 1990s but is occasionally also used as a solvent. It has a tetrahedral configuration with lead as the central atom. TinIV chloride 1M solution in heptane AcroSealR PubChem. Observing Electrochemical Reactions on Suspended Graphene.
 Source: sciencemadness.org
Source: sciencemadness.org
Thionyl chloride is primarily used as a chlorinating reagent with approximately 45000 tonnes 50000 short tons per year being produced during the early 1990s but is occasionally also used as a solvent. TinIV chloride solution 10 M in methylene chloride. The PbCl covalent bonds have been. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count. Thionyl chloride is an inorganic compound with the chemical formula S O Cl 2.
 Source: chemsrc.com
Source: chemsrc.com
Thionyl chloride is an inorganic compound with the chemical formula S O Cl 2. The PbCl covalent bonds have been. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count. Observing Electrochemical Reactions on Suspended Graphene. Dvir Gur Weizmann Institute of Science 1225-1245.
 Source: en.wikipedia.org
Source: en.wikipedia.org
TinIV chloride solution 10 M in methylene chloride. Chloride is determined by potentiometric titration with silver nitrate solution with a glass and silver-silver chloride electrode system. Thionyl chloride is an inorganic compound with the chemical formula S O Cl 2. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count. It is toxic reacts with water.

It is a moderately volatile colourless liquid with an unpleasant acrid odour. Property Name Property Value Reference. Thionyl chloride is primarily used as a chlorinating reagent with approximately 45000 tonnes 50000 short tons per year being produced during the early 1990s but is occasionally also used as a solvent. Thionyl chloride is an inorganic compound with the chemical formula S O Cl 2. Chloride is determined by potentiometric titration with silver nitrate solution with a glass and silver-silver chloride electrode system.
 Source: chemsrc.com
Source: chemsrc.com
It is toxic reacts with water. Property Name Property Value Reference. In the absence of interfering substances the precision and accuracy are estimated to be about 012 mg for 5 mg chloride or 25 of the amount present. Computed by PubChem 21 PubChem release 20210507 Hydrogen Bond Donor Count. Role of ammonium chloride surfactant headgroups Ran Eitan Abutbul Ben-Gurion University of the Negev.
 Source: sigmaaldrich.com
Source: sigmaaldrich.com
It has a tetrahedral configuration with lead as the central atom. Thionyl chloride is primarily used as a chlorinating reagent with approximately 45000 tonnes 50000 short tons per year being produced during the early 1990s but is occasionally also used as a solvent. It has a tetrahedral configuration with lead as the central atom. It is a moderately volatile colourless liquid with an unpleasant acrid odour. In the absence of interfering substances the precision and accuracy are estimated to be about 012 mg for 5 mg chloride or 25 of the amount present.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title tin iv chloride stability by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.